COVID-19
Good public health logistics for resilient health systems during the pandemic: Lessons from Tamil Nadu
Adithyan GS, Sundararaman T
Published online first on April 6, 2021. DOI:https://doi.org/10.20529/IJME.2021.026Abstract
The article highlights the importance of strengthening of public systems and the need for rapid scaling up of access to testing and to appropriate therapeutics in the context of the Covid-19 pandemic, to have in place robust public procurement systems for drugs and diagnostics. The paper draws lessons from the Tamil Nadu experience and validates the understanding that investing in public institutions is essential for rapid responsiveness to pandemics and other public health emergencies from both the ethical and health systems points of view.
Keywords: Covid-19, health systems, pandemic control.Background
Tamil Nadu (a southern state of India) has currently crossed 1.83 crore Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) testing and became the first State to do so in the country1. The cumulative testing rate for the State is, currently, above 200,000 RT-PCR tests per million, which is one of the highest testing rates across any of the larger States in India1. Tamil Nadu’s relative success in containment of the pandemic is considerably due to its testing strategy and the way it geared up public health services in the first few months, to ensure much better access to essential technologies for Covid-19 through its network of public health providers. When the lockdown restrictions eased, cases increased in June and July 2020, and peaked during mid-August. But from September onwards, the transmission curve flattened, and after three months at about 5000 cases per day, the number of cases started declining in mid-November and by February 2021 end, less than 500 cases were reporting per day1. In mid- March 2021, cases are slightly rising in Tamil Nadu with a similar trend seen in many states and in the country which is suggesting a second wave of infections1. The state’s mortality rate has remained limited to about 1.1% (0.7-1.9) throughout the last 4 months period, which is comparatively lower than bigger states like Maharashtra, Gujarat, West Bengal, Punjab2.
Overall, the monthly data analysis of the key Covid-19 indicators of the State as on, March 15, 2021 clearly shows the trajectory of the epidemic in Tamil Nadu (Fig 1). The comparison between five major southern states on the month-end active caseload from July 1, 2020 to March 15, 2021, when Rapid Antigen Tests (RATs) began to be widely used by States is depicted in Fig 2.
Figure 1: Monthly Covid-19 details, Tamil Nadu (as on 15.03.2021)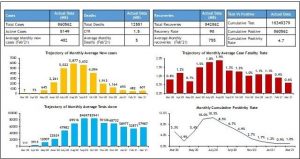
Source: Media Bulletins, Health and Family Welfare Department1 (Graphs prepared by authors)
Figure 2: Active cases-month wise: Comparison of five southern states (15.03.2021)
Source: www.covid19india.org2 (Graphs prepared by authors)
The choice of testing technology
Early in the epidemic, the State Government of Tamil Nadu adopted a policy of using only the RT-PCR, tests-considered the gold standard. Even when many other states shifted to Rapid Antigen Tests (RATs), Tamil Nadu preferred this option because it had a much lower rate of false negatives. Rapid antigen tests for Covid-19 are supposed to be comparatively cheaper and deliver quicker results than RT PCR, but are about 10 times less sensitive than the latter, leading to a high ratio of false negatives (1). Official Ministry of Health and Family Welfare (MoHFW) and Indian Council of Medical Research (ICMR) guidelines address this by calling for mandatory re-testing with RT-PCRs of all symptomatic patients who test negative with RATs (2). But this is difficult to organise and reports from many states show that this is not happening, even as per MoHFW sources (3). Such patients with missed diagnosis, who need to be re-tested, require to be isolated till then, and the failure to do so would be a major source of disease transmission. This also gives an inaccurate impression of the number of positive cases, because official figures do not disclose the numbers of symptomatic individuals who were not adequately tested. However, the advantages of RAT are that RT PCR machines require more complex logistical support and skilled manpower. The reported cost advantage of RATs during the initial period has since been negated, with the multiplicity of vendor options in RT-PCR and consequent competition driving down the RT-PCR costs, while the prices of limited vendor RAT kits has not come down commensurately.
The development of testing capacity
In March 2020, as the pandemic reached Tamil Nadu, it had only one laboratory with the capacity to test for Covid-19, the Kings Institute of Preventive Medicine & Research, Chennai, which was able to test about 90 samples/day. By April end, with the addition of more testing centres, the testing capacity had reached around 4500 tests/day. Currently as on March 15, 2021, this capacity rose to 257 testing centres of which 69 were in the government sector and 188 in the private sector ─ with all districts having at least one dedicated government lab for RT PCR testing1 (Figure 3). Aggressive and appropriate testing was the major strategy followed by the State during the pandemic period. The daily testing capacity is now almost 1 lakh RT-PCR tests per day in government institutions, which is the highest among all states. Though the number of government testing laboratories is less than half the number in the private sector, overall 78% of the test load in the State is carried out by the government RT-PCR laboratories and another 9% is covered under the Chief Minister’s Comprehensive Health Insurance scheme (in private labs) as per government reports3 (Figure 4a & 4b). This became possible because, government testing laboratories were functioning on a 24×7 basis with additional human resources supported by the state government to meet the increased testing capacity required. A total of around 550 laboratory technicians and 250 lab attendants were also appointed in this period3.
One major reason why utilisation of government testing facilities was higher was because this test, which costs about Rs 2500 per test in the regulated private sector, was made available free from all government hospitals.
Figure 3: Month wise – increase in RT-PCR laboratories in Tamil Nadu (15.03.2021)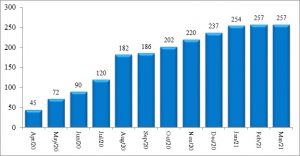
Source: Media Bulletins, Health and Family Welfare Department1 (Graphs prepared by authors)
Figure 4 a: Covid-19: Percentage share of government and private testing (as on 31.01.2021)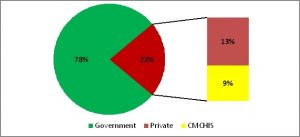
Source: State Control Room, Health and Family Welfare Department3 (Graphs prepared by authors)
Figure 4 b: Covid-19: Percentage share of government-funded free testing (including those covered under insurance) vs paid testing in private sector (as on 31.01.2021)
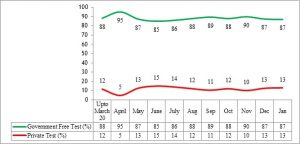
Source: State Control Room, Health and Family Welfare Department3 (Graphs prepared by authors)
The logistics of testing
The other major reason why the state could scale up testing to such a high frequency, is because of its excellent public health logistics. The logistics and supply chain management for these facilities were effectively managed by the Tamil Nadu Medical Services Corporation Limited (TNMSC), established way back in 1995.
The challenge is to maintain 69 well dispersed laboratories with the necessary kits, and a number of auxiliary consumables in the required quantities, though there would be wide variations in utilisation between them. Tamil Nadu’s ability to manage this logistical challenge rests a lot on its famed institution- the Tamil Nadu Medical Services Corporation which was able to ensure uninterrupted and adequate supply of nasal swabs, viral transport medium (VTM), extraction kits, RT-PCR kits, reagents, and other consumables. The TNMSC has so far procured and supplied more than 1 crore kits as on January 31, 20213. These testing kits, and the equipment were new products with limited manufacturers and one had to have transparent procurement that brought in high quality test kits, but at very low procurement costs, since the entire testing expenditure was being borne by the state.
Technology choices adopted in government RT PCR labs
In addition to establishing new labs, the other key factor which ensured the success of the testing strategy implemented through public institutions is the choice of technology adopted by TNMSC. The first was the drive to automate all the government labs for the RNA extraction process, since this was the most time-consuming part of the testing cycle. This was achieved by ensuring that automated extractors were installed in all labs and today, no government lab does manual extraction. This helped to eliminate employee fatigue and enabled the state to sustain high rates of testing for several months. Second, the insistence on performance parameters for RT-PCR kits with strict adherence to international quality norms as well as assay time of 45-50 minutes helped the state government to drastically reduce dependence on private labs.
The above strategy has a significant economic advantage too, considering the difference in cost of testing between the ramped up public institutions’ capacity and Tamil Nadu’s well developed private sector. At present, even after multiple reduction mandates by the state government, the prescribed rate for RT-PCR testing of government samples in Tamil Nadu’s private labs is Rs. 1200 as against Rs. 360 in government labs (provided free of cost to patients). This includes the additional costs of automated RNA extraction and kits with international certifications. Even if the initial costs are capitalised, the cost per test is likely to be only around Rs. 400. This cost saving has been the primary factor in Tamil Nadu’s ability to sustain its “only RT-PCR testing strategy”.
TNMSC also made the technology choice to opt for open systems of hardware, which are compatible with diverse consumables rather than closed systems, which offered higher throughput at higher recurring costs. To improve throughput in such open system hardware, equipment was supplied to automate the crucial steps in testing, and the orders for such hardware were strictly tied to guarantees of consumable supplies.
Access to therapeutics
Tamil Nadu’s health system has been described as one of the better health systems in the country, with a much better performance on many key health indicators (4). The Directorate of Public Health and Preventive Medicine, functioning since 1923, has the experience of both public health functions and the organisation primary healthcare till First Referral Units or Community Health Centres. Its position with respect to health professionals is relatively better than other states.
One of the challenges that even good health systems face in responding to the Covid-19 pandemic is the huge expansion of hospitalisation capacity that is required. Though increased numbers of beds and staff is often what is most visible, equally important is the supply chains required for ensuring that the necessary equipment and consumables are in place. The presence of an innovative public institution like Tamil Nadu Medical Services Corporation (TNMSC) made a huge difference to Tamil Nadu’s health systems preparedness during this pandemic. The TNMSC has a good reputation built over two decades of how a highly effective and efficient supply chain management can be ensured for delivery of public services, where all the drugs and diagnostics are provided free to patients. Its success had been attributed to its relative autonomy that enables a professional leadership to develop and provides considerable operational flexibility, a high level of transparency, its negotiation of rates and fixing of rate-contracts, and processes that make drug distribution responsive to changing demand patterns. This flexibility helped TNMSC take swift action in February 2020, and even before the pandemic wave reached Tamil Nadu, the TNMSC had built up stocks in the district warehouses of all essential drugs required in each district for the next six months3. This was done anticipating supply chain disruptions considering that the epidemic was then centred in China, the principal supplier of Active Pharmaceutical Ingredient (APIs) to India’s generic drug industry. Further, in March 2020, faced with the challenge of ordering a number of new products, the TNMSC has adopted, under clause 16 (A) of the Tamil Nadu Tender Transparency (TT) Act, the suspension of key provisions of the Act as is permissible during natural calamities and emergencies. This enabled the Corporation to do rapid procurement of essential new drugs, consumables and equipment in the changed supply side conditions, where there were few or no providers. The TNMSC took it on itself to forecast the demand under the changed circumstances, and procured medicines in anticipation of formal orders and payments. On some commodities, the TNMSC made up to 50% advance payments, so as to ensure its supply as against the usual practice of full payment only after delivery. When those rate-contracted firms ran out of supplies, TNMSC could go to the next available supplier and negotiate the next best deal. To ensure cash flows, interest free advances were also issued against indemnity bonds so that the vendors down the supply chain were given the necessary confidence. There were further challenges in procuring items from other countries, and diplomatic channels had to be leveraged to ensure quality supply.
Another major challenge was the supply of medical oxygen. The TNMSC estimated requirements for three types of supply: liquid medical oxygen tank capacity for the tertiary care institutions and other large public hospitals, D-type bulk cylinders for all institutions and B-type cylinders for emergency use in Covid Care Centres (CCCs). In addition it also provided for a large number of portable oxygen concentrators as back-up. The supply chain mechanism was able to ensure that no oxygen bed went short of oxygen when they required it. In March, when the pandemic broke, both the public and private sectors had almost equal liquid medical oxygen capacity ─ about 355.1 kilo litre in public and 314.6 kilo litre in private3. By October, this had increased to 519.4 in Public sector, whereas it remained stagnant in the private sector3. The plan is to enhance this to 834.11 KL and work is on to achieve this capacity3. The number of Type D cylinders deployed rose from 4649 to 6649 and Type B cylinder capacity from 4893 to 65433. Such rapid increases in capacity required close collaboration with all the concerned government departments and the private manufacturers and distributors.
The number of “oxygen beds” (including ICU beds) increased from less than 300 in March 2020 to approximately 22,000 in October, 2020, in public facilities. Similarly, the number of ventilators in public facilities reached almost 6000, wherein more than 50% of procurement was done during the last eight months. The numbers of High Flow Nasal Cannula (HFNC) and Continuous Positive Airway Pressure Therapy (CPAP) units in the facilities during March 2020 were 85 and 124 respectively, and were increased to 2164 and 201 respectively, by October 20203. The entire procurement and installation of the equipment with all the necessary consumables for this was again the work of the TNMSC. The availability of trained manpower, especially in nursing care in TN’s public sector hospitals as compared to other states also enabled the state to reap better health outcomes out of the better oxygen supply and access to other therapeutics.
There were also no pricing issues in the Tamil Nadu public health system since the government had a clear commitment to provide all these hospital services free of charge, including medication, diagnostics and ICU bed charges, and they also capped the rates for private institutions (5). The rate contract for all drugs was finalised by TNMSC and the requirement of each district was directly delivered to dedicated district warehouses. To avoid disruptions in availability, a model of regular releases to institutions was also formulated by TNMSC. The entitlement of each health institution was fixed based on its bed strength and cases. The institutions were allowed to lift the items once every two days from the warehouses without any payment, by accounting for all the Covid-19 related supplies as of zero value, and not linking them to their corresponding regular budgetary allotments.
TNMSC ensured adequate availability of special high end drugs such as Tocilizumab Injection, Remdesivir, Enoxaparin Injection for the management of Covid-19 in all government medical institutions throughout the state by adopting a streamlined and centralised procedure for procurement, storage and distribution. To counter the challenges that may arise during the procurement and supply of drugs, competitive pricing was offered to the companies for procurement. A recent government report states that as of Nov 15, 2020, TNMSC had supplied 4,63,344 vials of Remdesivir Inj and 8,31,969 vials of Enoxaparin Inj and 2047 of Tocilizumab Inj. Dexamethasone of course was already part of the government supply chain3.
Since the scientific understanding and clinical management of the pandemic keep evolving day by day, procurement policies also need to be fine-tuned in such a manner as to ensure supplies in a sellers’ market. TNMSC continuously followed USFDA approvals and other developments on therapeutic applications in this regard. Early large scale adoption of Remdesivir, cautious adoption of Tocilizumab and avoiding Favipiravir, installing CT scans earmarked for Covid patients etc. were a few examples of the successful evidence –based procurement planning carried out by TNMSC.
Need for a public procurement and logistics agency in healthcare: An ethical standpoint
Almost a year after the start of the epidemic, the transmission curve has shown a declining trend till February 2021 and a slight increase in cases currently in March 2021, suggestive of a second wave. There are many reasons for Tamil Nadu’s relatively good performance throughout the course of the pandemic. But what we highlight in this paper is how rapid scaling up of access to testing and to appropriate therapeutics requires robust public procurement systems for drugs and diagnostics to be already in place; both from a health systems strengthening point of view as well as an ethical standpoint ─ especially when the whole country is currently witnessing a second surge of infections. In a context where the entire treatment has to be provided without any user fees, a good public procurement agency for drugs and diagnostics brings value for money and upholds the value of equity. TNMSC officials claim that Tamil Nadu has managed the lowest rate in the country for all the consumables in use, while ensuring quality assurance and deploying the best technology options. This is one reason why Tamil Nadu has been able to reach one of the highest levels of testing and institutionalised care for Covid-19 in the country with very low out-of-pocket expenditure (OOPE) for needy patients.
The lack of a public procurement agency, especially in healthcare, results in both shortages and wastage and affects access to tests and therapeutics. It can also lead to inaccurate quantification, delays in tendering decisions, payment delays and inadequate monitoring and finally affecting healthcare for the poor (6). Hence, it is the need of the hour from an ethical standpoint to build strong public procurement and logistics agencies in line with TNMSC for drugs and diagnostics, not just because both are the most important contributors for OOPE, but also for providing quality driven effective care addressing equity.
Covid-19 has exposed both the vulnerabilities and opportunities in public health systems worldwide. One measure of the success of any public service institution is gauged by its efficiency, responsiveness and resilience, when faced with a crisis. The Tamil Nadu experience shows that public trust in public health institutions is still intact. It validates the understanding that investing in public institutions is essential for responsiveness to pandemics and other public health emergencies, not just from a health systems perspective but also an ethical imperative. A robust public health procurement and logistics system is an essential element of building both trust and resilience in public health services. These are lessons for every state. But states like Tamil Nadu should go further and also invest in developing domestic manufacturing capacity and building the innovation ecosystems required for the necessary medical products ‒ including vaccines, diagnostics, devices, and personal protective equipment.
Acknowledgement: Dr P Umanath and the team from TNMSC provided critical inputs which helped the authors substantially in developing the article. The authors wish to acknowledge all staff from TNMSC, State Emergency Operations Center (EOC), Tamil Nadu-Health Informatics Division, National Health Mission and other Directorates, who gave us their time for detailed information and shared data from internal review reports. We are also grateful to the Department of Health and Family Welfare, Tamil Nadu for the permission to publish the same. The views expressed in this paper and responsibility for errors if any, are however with the authors.
Notes- 1. Most of the data mentioned in the paper is from the Daily Media Bulletins published by Health & Family Welfare Department, Tamil Nadu.( https://stopcorona.tn.gov.in/daily-bulletin/). Last accessed March 15 2021.
- 2. www.covid19india.org (website) Last accessed March 15 2021.
- 3. The data on TNMSC procurement of drugs, diagnostics, oxygen capacity, beds, ventilators etc. was obtained from Internal Reports from TNMSC and the Health & Family Welfare Department, Tamil Nadu
References
- Mak GC, Cheng PK, Lau SS, Wong KK, Lau CS, Lam ET, Chan RC, Tsang DN. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin Virology. 2020 Jun 8:104500.
- Indian Council of Medical Research (ICMR). Advisory on Strategy for COVID-19 Testing in India (Version VI, dated 4th September 2020[cited 2020 Nov 5] Recommended by the National Task Force on COVID-19. Available from: https://www.mohfw.gov.in/pdf/AdvisoryonstrategyforCOVID19TestinginIndia.pdf
- PTI. Health ministry asks states, UTs to mandatorily retest all symptomatic RAT negative cases. Times of India. 2020 Sep 10[cited 2020 Nov 5]. Available from: https://timesofindia.indiatimes.com/india/health-ministry-asks-states-uts-to-mandatorily-retest-all-symptomatic-rat-negative-cases/articleshow/78035679.cms
- Das Gupta M, Desikachari BR, Shukla R, Somanathan TV, Padmanaban P, Datta KK. How Might India’s Public Health Systems Be Strengthened? Lessons from Tamil Nadu. Econ Pol Wkly. 2010 Mar 6[cited 2020 Nov 5];45(10):46-60.
- Health and Family Welfare (EAP 1-I) Department, Tamil Nadu. G.O. (Ms). (No). 240. 2020 Jun 5 [cited 2020 Nov 25]. Available from: https://cms.tn.gov.in/sites/default/files/go/hfw_e_240_2020.pdf
- Chandra Sharma N. What ails India’s public health system? Livemint. 2017 Sep 20 [cited 2021 Jan 20]. Available from: https://www.livemint.com/Politics/JaTFUOPjyMB2Yu86LjwAAJ/What-ails-Indias-public-health-system.html
