COMMENT
CTRI requirement of prospective trial registration: Not always consistent
Indraneel Chakraborty, Gayatri Saberwal
Published online first on May 13, 2022. DOI:10.20529/IJME.2022.033Abstract
In a clinical trial registry, one determines whether a trial is registered prospectively or retrospectively by comparing the date of registration with the date on which enrollment started. However, in Clinical Trials Registry – India (CTRI), in addition, the top of each record is labelled with the phrase “Trial Registered Prospectively” or “Trial Registered Retrospectively”. In examining CTRI records, we have found that (a) although retrospective registration has been disallowed from April 1, 2018, some trials were registered retrospectively; (b) in some cases, enrollment started after registration, even though they were labelled “Trial Registered Retrospectively”, which is misleading; and (c) in some cases, the date of first enrollment was modified, changing a retrospective registration to a prospective one, although the label “Trial Registered Retrospectively” persisted. This, too, is misleading. The CTRI administration should take suitable steps to prevent late registration and mislabelling of trials regarding their registration status.
Keywords: Clinical Trials Registry – India, ethics, data integrity
Since the year 2000, an increasing number of national and regional clinical trial registries have been established. Today, 18 registries act as data providers to the International Clinical Trials Registry Platform of the World Health Organization (WHO), and Clinical Trials Registry – India (CTRI) is one of them [1].
Over these years, world-wide, various stakeholders such as regulators, academics, activists, journals, WHO, philanthropic funders, ethics committees etc, have pushed for (i) trials to be registered in a public trial registry [2]; (ii) data entry for each trial record to be correct [3, 4]; (iii) data related to a given trial to be consistent across various sources such as registries, publications and regulatory documents [5] and so on. An incorrect or incomplete public record of all trials and their results, would be unethical, since it would breach the social contract with the trial participants [6]. It is also likely to contribute to research waste [6], that is, future research cannot be built on such trials, and therefore, there is limited societal benefit of such trials. Further, it might create bias in the literature, as discussed below.
For decades, it has been known that clinical trials with positive results are more likely to be both submitted and accepted for publication [7]. This publication bias skews the literature, which is harmful for efforts to advance evidence-based medicine. If all trials were registered, then a trial could not be hidden, since any interested person could follow up with the sponsor regarding a registered trial, even if the results were not in the public domain. Another well-known problem is that the investigator may redefine the primary and secondary outcomes after taking a look at early results, and claim an outcome that happened by chance as a definitive outcome, and a proof of efficacy of the candidate drug, for instance [8]. This can be done in a retrospectively registered trial, when there is time to change the primary or secondary outcomes before registration. However, it is unlikely to be done in a prospectively registered one, unless there is very good justification [9].
Several primary registries of the WHO mandate prospective registration of trials [1]. CTRI has required this since April 1, 2018. The best method to determine whether a trial is registered prospectively or retrospectively is to compare the date of registration with the date on which enrollment started. In the case of CTRI, the latter has two versions, that is, “Date of First Enrollment (India)” and “Date of First Enrollment (Global)”. In addition, the top of each CTRI record is labelled, “Trial Registered Prospectively” or “Trial Registered Retrospectively”.
Our review of the CTRI database after April 1, 2018 has shown that some trials have not been registered prospectively, as required. Of the 18,514 trials that were registered with CTRI between April 1, 2018 and February 19, 2021, 1261 (6.8%) were labelled “Trial Registered Retrospectively” [Figure 1] and [Supplementary File 1, available online only]. On examining these records, we found that most trials had started recruiting before April 1, 2018. There was a huge jump in the number of registrations in the lead up to this deadline [4] and in view of the large number of submissions, the registry accepted applications if enrollment had started before April 1 [10].
Figure 1: Different categories of trials labelled “retrospectively registered”
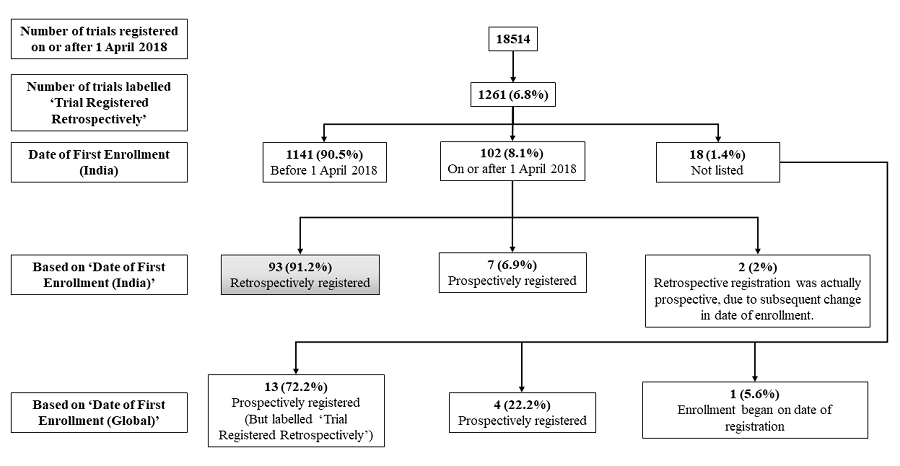
However, 102 records of “Trial Registered Retrospectively” cases had the “Date of First Enrollment (India)” on or after April 1, 2018. In addition, 18 trials had no data for the “Date of First Enrollment (India)”.
We first examined the set of 18 foreign trials, none of which intended to recruit from India. In four cases, the date of registration was after the “Date of First Enrollment (Global)”. However, the latter date was before April 1, 2018. In one case, recruitment started on the day of registration. These five trials, had principal investigators based in Bangladesh (n = 1), Nepal (n = 3) and Poland (n = 1). We need not focus on these five trials. In 13 cases, enrollment started after registration, even though they were labelled “Trial Registered Retrospectively”. These trials had principal investigators in Bangladesh (n = 6), Egypt (n = 1), Nepal (n = 3) and Saudi Arabia (n = 3). In these 13 cases, the “Trial Registered Retrospectively” label has caused confusion, since the trials were prospectively registered. In none of these cases did the investigator change the date of enrollment subsequently, and therefore this mislabelling appears to have existed since the trial was registered.
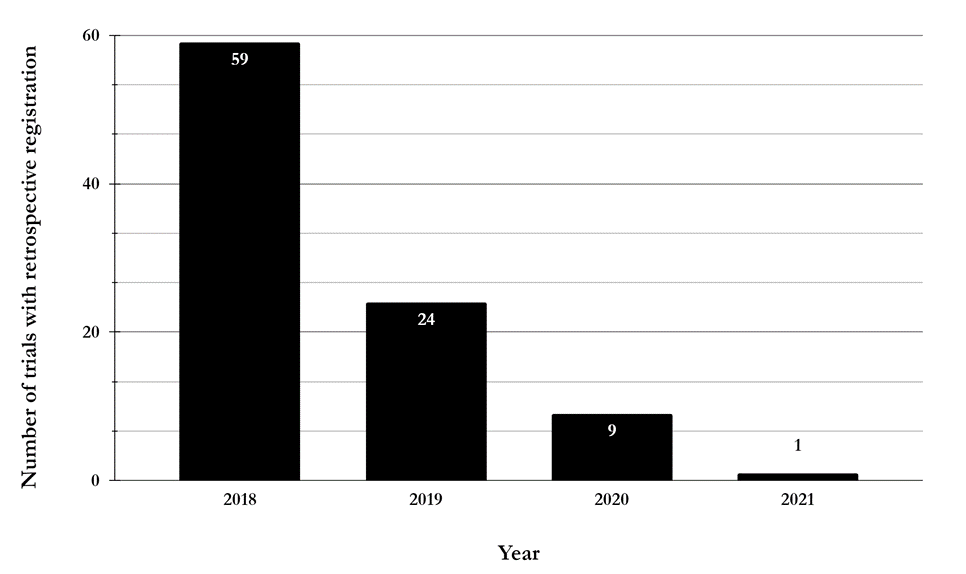
Of the 102 cases, a comparison of the date of registration and the “Date of First Enrollment (India)” indicated that, in fact, nine trials were prospectively registered. In two of these nine cases, the date of first enrollment had been modified after trial registration, presumably to reflect a change from “estimated” to “actual” date of enrollment. Therefore, at the time of registration they were retrospective cases, although, subsequently they became prospective cases. It appears that the “Trial Registered Retrospectively” label was based on the estimated rather than the actual date of first enrollment. However, the other seven did not undergo any such modification, so it is surprising that they were labelled retrospective cases. The remaining 93 trials were genuinely retrospectively registered. In terms of timelines, the breakup of these 93 cases was as follows — 59 (63.4%) trials started recruiting in 2018, 24 (25.8%) in 2019, 9 (9.7%) in 2020, and one (1.1%) in 2021 [Figure 2]. This indicates that although the number of cases is low, the problem of genuine retrospective registration persists even today.
Figure 2: Number of retrospectively registered trials per year
In terms of percentages, 1261 (6.8% of 18,514) trials appeared not to have followed the provision that required prospective registration since April 1, 2018. However, if we only consider cases that started enrollment after April 1, 2018, only 93 were retrospectively registered based on the “Date of First Enrollment (India)”. In addition, based on the “Date of First Enrollment (Global)”, 13 were incorrectly labelled “Trial Registered Retrospectively”. If we only consider the 93 cases that were genuinely registered retrospectively, these amount to 0.5% of 18,514 cases. Although the fraction of trials that breaks the prospective registration requirement, is minuscule, and decreasing over time, it is nevertheless worth pointing out, since a small problem has the potential to become a bigger problem in future.
To conclude, the registration of Indian trials in CTRI has been mandated by the Central Drugs Standard Control Organisation (CDSCO) [11], by journals [12] and by ethics committees [13]. Many of them require prospective registration, which contributes to fulfilling an ethical obligation to trial participants, and will reduce publication bias and research wastage. Nevertheless, some trials have been registered retrospectively. In earlier work, we have proposed that CTRI should implement logic rules to prevent certain types of errors [4]. Such rules could prevent the errors identified here. We urge the CTRI administration to take suitable steps to prevent such inaccuracies and misleading data entries. As a first step, it would be helpful if the current mislabelling were corrected.
Funding support: This work was supported by the Department of Electronics, IT, BT and S&T of the Government of Karnataka.
References
- Venugopal N, Saberwal G. A comparative analysis of important public clinical trial registries, and a proposal for an interim ideal one. PLOS ONE. 2021 May 11;16(5): e0251191. https://doi.org/10.1371/journal.pone.0251191
- Krlezˇa-Jeric K, Chan A-W, Dickersin K, Sim I, Grimshaw J, Gluud C et al. Principles for international registration of protocol information and results from human trials of health related interventions: Ottawa statement (part 1). BMJ. 2005 Apr 23;330(7497): 956-8. https://doi.org/10.1136/bmj.330.7497.956
- Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov Results Database — Update and Key Issues. N Engl J Med. 2011 Mar 3;364(9): 852–60. https://doi.org/10.1056/NEJMsa1012065
- Pillamarapu M, Mohan A, Saberwal G. An analysis of deficiencies in the data of interventional drug trials registered with Clinical Trials Registry – India. Trials. 2019 Aug 28;20(1): 535. https://doi.org/10.1186/s13063-019-3592-0
- Pradhan R, Singh S. Comparison of Data on Serious Adverse Events and Mortality in ClinicalTrials.gov, Corresponding Journal Articles, and FDA Medical Reviews: Cross-Sectional Analysis. Drug Saf. 2018 Sep;41(9): 849–57. https://doi.org/10.1007/s40264-018-0666-y
- DeVito NJ, Bacon S, Goldacre B. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study. Lancet. 2020 Feb 1;395(10221): 361–9. https://doi.org/10.1016/S0140-6736(19)33220-9
- Dickersin K, Rennie D. Registering clinical trials. JAMA. 2003 Jul 23;290(4): 516–23. https://doi.org/10.1001/jama.290.4.516
- Dal-Ré, R., Ross, J. S., & Marušić, A. Compliance with prospective trial registration guidance remained low in high-impact journals and has implications for primary end point reporting. J Clin Epidemiol. 2016;75: 100–107. https://doi.org/10.1016/j.jclinepi.2016.01.017
- Goldacre B, Drysdale H, Dale A, Milosevic I, Slade E, Hartley P, et al. COMPare: a prospective cohort study correcting and monitoring 58 misreported trials in real time. Trials. 2019 Feb 14;20(1): 118. https://doi.org/10.1186/s13063-019-3173-2
- Clinical Trials Registry of India. Important Notice for all Trial Registrants. Date unknown [cited 2021 Sep 17]. Available from: http://ctri.nic.in/Clinicaltrials/alert.php
- Clinical Trials Registry of India. About CTRI. Date unknown [cited 2021 Sep 17]. Available from: http://ctri.nic.in/Clinicaltrials/cont1.php
- Pandey A, Aggarwal A, Maulik M, Seth SD. Clinical trial registration gains momentum in India. Indian J Med Res. 2009;130: 85–6.
- Tharyan P. Ethics committees and clinical trials registration in India: opportunities, obligations, challenges and solutions. Indian J Med Ethics. 2007 Oct-Dec;4(4): 168–9. https://doi.org/10.20529/IJME.2007.066
