RESEARCH ARTICLE
Association of funding and conflicts of interest on outcomes reported in published studies of Covid-19
Snehalata Gajbhiye, Chaitali Chindhalore, Ashish Gupta, Ganesh Dakhale
Published online first on March 10, 2023. DOI:10.20529/IJME.2023.022Abstract
Background: An outbreak of the Covid-19 has led to substantial mortality globally. The entire world is carrying out studies to understand the pathophysiology, clinical features, diagnosis and treatment of Covid-19. We investigated the possible association of type of funding, corporate or academic, and conflict of interests on the outcomes reported in clinical trials on Covid-19.
Methods: Studies containing the keywords “clinical trial” AND “Covid 19” or “Corona” were located by a search on PubMed published between September 2019 to August 2021. Filters were used to select only papers in the English language and on “humans”. The data were analysed using descriptive statistics and the Chi-square test.
Results: We found a significant association between the existence of a conflict of interest and reporting of a positive outcome (X2 value = 18.751, p<0.001). We also found a significant association between industry funding and reporting of a positive outcome (X2 value = 18.041, p<0.001).
Conclusion: We conclude from this study that the presence of conflict of interest and pharmaceutical industry funding is associated with reporting a positive outcome.
Keywords: PubMed, pandemic, clinical trials, industry funding, conflict of interest
Introduction
In late December 2019, a disease outbreak due to a novel coronavirus began in Wuhan China and quickly spread across the globe. The epidemic was declared a pandemic by the World Health Organization (WHO) on March 12, 2020 [1]. Globally, as of Feb 2023, it is found to have caused seven hundred million confirmed cases and over six million deaths worldwide [2]. Numerous pharmaceutical companies and academic institutes began racing to find effective therapies for the treatment and prevention of SARS-CoV-2 across the world, including in India. Worldwide, several therapies received regulatory approval comprising antiviral remdesivir, remdesivir plus baricitinib, dexamethasone, convalescent plasma, bamlanivimab and the fixed-dose combination of casirivimab plus imdevimab [3, 4]. Also, several vaccines got regulatory approval from different countries and have been administered across the globe. Despite these developments, effective treatment options for Covid-19 remain limited. The large quantity of clinical data being generated, a wide spectrum of disease presentations, and rapid mutations presented a critical need for analysing the data generated from an ethical point of view.
Several non-Covid studies in the literature have demonstrated an association between funding from pharmaceutical companies and the presentation of positive findings [5, 6, 7]. There are also studies in the non-Covid area which have shown an association between conflicts of interest (COI) and the presentation of positive findings [6, 7]. A huge number of studies have been published during the pandemic. Available evidence suggests that the methodological quality of studies on Covid-19 is poor [8]. According to Jung et al, Covid-19 clinical studies have a shorter processing time from submission to publication and have lower methodological quality scores than control studies in the same journal [8]. The scientific integrity of the clinical trial data is also open to question. An expert commentary by Dinis-Oliveira et al highlights the importance of scientific integrity during the SARS-CoV-2 crisis and aims to alert health professionals, that they must not blindly trust the findings, even if the journal has a high impact factor [9].
Against this background, we decided to evaluate studies for the type of funding and conflict of interest and study their association with the outcome (positive or negative) in published studies of Covid-19.
Materials and methods
This was a retrospective study carried out over a period of three months on data extracted from studies available in the public domain.
Ethics approvalThe ethics approval for the study was obtained from the Institutional Ethics Committee, All India Institute of Medical Science, Nagpur
Search strategyPubMed/MEDLINE was searched using the keywords “clinical trial” AND “Covid 19” or “Corona”, individually by two authors and confirmed by the senior author.
Selection criteriaStudies in “English” language on “human” species published from September 2019 to August 2021 were included. The study was executed (data collection and analysis) from September to October 2021. All clinical drug trials whether therapeutic or prophylactic published during the relevant period were studied.
Published study protocols, non-drug trials, trials with a single-arm, observational studies, studies on medical education on Covid-19, retracted articles and randomised controlled trials, where data is missing were excluded.
As both therapeutic and prophylactic trials were included, the population comprised of healthy volunteers (in vaccine and other prophylactic intervention trials) and patients suffering from Covid-19. Also, the studies were selected irrespective of age group, thus, the population comprised of both paediatric and adult age groups. The drug trials of Covid-19 were selected, thus the intervention comprised of drug/vaccine. The comparator included participants who were on the standard of care or placebo or no therapy control arm. The type of outcome assessed was the reporting of the positive or negative outcome. The study design chosen was randomised and nonrandomised interventional studies.
Outcome measuresThe primary outcome measures were: a) the comparison of positive and negative outcomes in pharmaceutical vs non-pharmaceutical funded studies and b) the comparison of a positive or negative outcomes in studies with and without conflict of interest. The secondary outcome measures were the proportion of studies with different types of intervention, the proportion of studies with therapeutic or prophylactic intervention, the proportion of studies with different types of study design (arms, randomized, blinded), the proportion of studies with different types of funding, the proportion of studies with different types of COI.
DefinitionsConflict of interest
There is no universally accepted consensus for any single definition of COI. Cancer Research UK has listed eight types of relationships between investigators and sponsors that constitute a COI and these are as follows:
• Employment, directorship, or leadership position
• Advisory role (paid or unpaid)
• Stock ownership or options
• Any other direct or indirect financial interest (eg, via rewards to inventors)
• Honoraria payments for specific speeches, seminar presentations, or appearances
• Research funding
• Expert testimony
The published study was considered to have a conflict of interest if the authors state any of the above-mentioned roles/ associations.Sources of funding
We categorised the sources of funding based on the information reported in the published papers. The source of study funding was determined by published disclosures. If the sponsors or collaborators included representatives of the industry the study was categorised as “pharmaceutical industry-funded”. All other types of studies were classified as “non-pharmaceutical industry-funded studies”. We searched the author’s affiliation, the methods section, sources of support or acknowledgments in the paper for the information.
Outcome definitions
• Positive: If the drug trial showed the primary objective to be statistically significant as compared to the control arm, the trial was termed positive.
• Negative: If the drug trial showed the primary objective not statistically significant as compared to the control arm, the trial was termed as negative.
Review by authors
Two investigators reviewed the studies and matched the results. The review of studies by two investigators reduced the chances of errors. The entered data were compared for any disagreement. Disagreement is defined as any of the investigators having a difference of opinion regarding the completed entries. Disagreement were sorted after discussion and deliberation with the help of the third and fourth authors and a consensus was reached for all the entries.
Statistical AnalysisBoth descriptive and inferential statistics were applied to the data. The categories of funding and conflict of interest were described in frequencies and percentages (descriptive statistics). The association of outcome (positive or negative) with the presence or absence of pharmaceutical funding and presence or absence of COI was done using a Chi-square test with a crude odds ratio (cOR) [with 95% Confidence Interval (CI)]. All analyses were done at 5% significance using GraphPad Prism 7.
Results
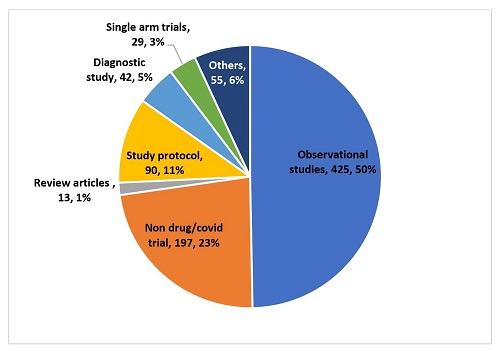
The total number of studies found on applying the keywords and filters was 1,043. The process of selection of studies is depicted in Figure 1. All studies were screened and the number of studies that satisfied the selection criteria was 192 (18.40%). The reasons for exclusion are depicted in Figure 2. Of the 851 studies excluded, the most common reasons for exclusion were their being observational studies (425, 50%) and non-drug or non-Covid trials (197, 23%).
Figure 1: Flowchart showing studies excluded at different stages
Figure 2: Studies that were excluded along with the reasons
Of the 192 studies, 102 (53%) were multicentric and 90 (47%) were single-centre studies. There were 179 (93%) studies with a randomised study design and 13 (7%) with no randomised study design. Of the 192 studies, 111 (58%) had an open-label design, 69 (36%) had a double-blind design, 6 (3%) had a single-blind design, and the remaining 6 (3%) had a triple-blind or observer-blind design. The standard of care comparative arm was taken in 101 (53%) studies, placebo-control studies were 83 (43%) and others were 8 (4%) which included only preventive advice etc. There were 158 (82%) studies where the intervention studied was therapeutic in nature and 34 (18%) were prophylactic. The outcome was reported as positive in 104 (54%) studies and reported as negative in 88 (46%) studies.
Type of funding and conflict of interestFunding was found to be from the pharmaceutical industry in 41 (18.75%) studies, while the remaining 151 (81.25%) studies were funded by non-pharmaceutical sources. Eighty-one (42%) studies reported the presence of COI and 111 (58%) reported the absence of COI. Figure 3 shows the type of conflict of interest in the clinical trials of Covid-19. The total of 1,221 authors reported COI. Of these, 452 (37.01%) authors reported conflict of interest by way of research grants. While 232 (19%) reported being employees of the pharmaceutical industry, 203 (16.62%) reported receiving honoraria as professional or consulting fees from the pharmaceutical industry. Of 1221, 103 (8.43%) authors reported having stock options of the pharmaceutical companies, and 99 (8.10%) reported having an intellectual property right (IPR) in the molecule tested.
Figure 3: Number of authors reporting various types of conflicts
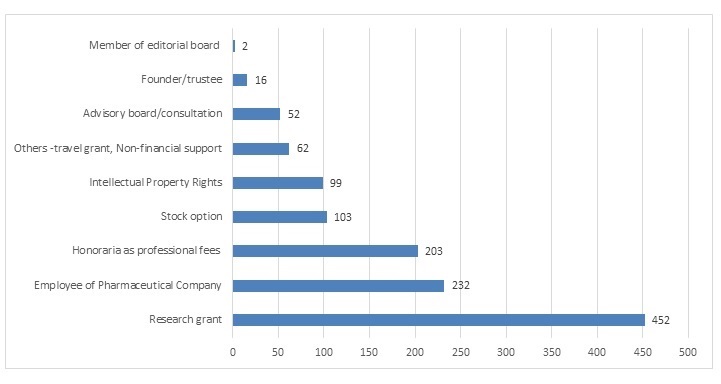
Table 1 shows a significant association between the presence of COI and reporting of positive outcomes (cOR = 4.11, 95%CI = 2.23-7.55, p<0.001). Similarly, there was an association between the absence of COI and reporting of negative outcomes. We classified the studies as “pharmaceutical” and “non-pharmaceutical funded”. We observed a significant association between reporting of pharmaceutical funding and positive outcomes [cOR = 3.76, 95%CI = 1.78-1.96, p<0.001].
Table 1: Association between types of funding and COI with outcome
Outcome |
|||||
Positive |
Negative |
X2 value |
P value* |
||
| COI | Yes | 53 (27.60%) | 28 (14.58%) | 21.677 |
<0.001 |
| No | 35 (18.22%) | 76 (39.58%) | |||
| Funding | Industry funded | 29 (15.10%) | 12 (6.25%) | 13.018 |
<0.001 |
| Non industry funded | 59 (30.72%) | 92 (47.91%) | |||
Discussion
We observed that the most common study design was an open label, randomised, standard care-controlled study. The contribution from non-pharmaceutical sectors (including the government, private and other funding programmes) in Covid-19 related research is higher. The studies reporting negative results are as many as those with positive results. The presence of COI and pharmaceutical industry funding is associated with reporting of a positive outcome.
Many treatment strategies have been evaluated for treatment alternatives for Covid -19 including antiviral drugs, anti-inflammatory drugs and drugs with immunomodulatory actions. Our findings are similar to those of other authors who found that the majority of trials assessing therapeutic strategies, are randomised and open-label in design [10, 11]. This underlines the importance of carrying out more studies with better methodological designs such as blinded clinical trials for handling bias in the conduct of research studies. Most of the studies included by us used the established standard of care as a comparative arm and most trials included were therapeutic. This finding is similar to the study published by Wang et al [11]. We did not find any study that has analysed the outcome of Covid-19 studies. Our study has shown that both the positive and negative outcomes have been reported equally in published clinical trials of Covid-19. This highlights the fact that the studies with negative outcomes were published by journals considering them as important evidence.
The result of our study is in agreement with Mehta et al who found similar funding sources for Covid-19 trials [12]. This is a welcome finding as unlike with other disease conditions — where industry funding is a major source for conducting clinical trials — we found funding from other sectors [7]. At the time of an emergency/pandemic, it is the responsibility of all stakeholders to contribute resources equally to generating data. Primary comparisons while analysing the impact of funding source on the outcome, were examined for the industry- versus non-industry-supported studies. COI is also an important aspect that requires attention. COI could exist during the entire process right from initiation of the study to publication, this has only been recognised and addressed by the scientific community in the recent past. In our study, 42% of the studies covered revealed disclosure of COI by at least one author. More journals need to demand full disclosure of COI, which is already a requirement with most high-impact journals as per the guidelines set by the International Committee of Medical Journal Editors, the World Association of Medical Editors, and the Committee on Publication Ethics [13].
Since the start of the pandemic, there have been a huge number of publications around Covid-19. The large volume of these articles itself raises questions on the quality of the peer-review process [14]. During a pandemic, the likelihood of deliberate misconduct cannot be ruled out [15]. Thus, authors need to be very transparent in declaring their conflicts of interest to establish trust in the public regarding their findings. There is a viewpoint that such disclosures should go beyond just authors and include disclosures from reviewers and editors [16]. These factors prompted us to study whether there is an association between the disclosures of COI and reporting of outcomes. We found a significant association between disclosures of COI and reporting of a positive outcome. We could not find a similar study in the literature that is conducted on studies carried out during the pandemic. However, we found a few studies in different areas that studied the association between conflict of interest and outcomes reported. Amiri et al found that there was no association between self-reported conflict of interest and study outcome around spinal research [17]. According to Perlis et al, among the 162 randomised, double-blind, placebo-controlled studies examined in Psychiatry research, those that reported conflict of interest were 4.9 times more likely to report positive results [7]. In yet another study, the existence of either financial or professional conflict of interest was significantly associated with favourable study outcomes [6].
The conflicts reported in a study may be viewed as a risk to research integrity and a barrier to good science, and may result in a loss of public trust [18]. The fact of researchers with conflicts of interest has given rise to conflicting views among the medical fraternity, wherein one group acknowledges that bias is not necessarily present when COIs are disclosed [19]. Also, there is a concern that the policies evolved for management of this COI may themselves restrain innovation and delay the process of laboratory to bedside benefit [19]. On the other hand, some groups advocate separation of researchers from industry as COI may adversely affect science [20]. However, we must note that the presence of COI will not help us to know the degree of influence by the researcher and also, we will never come to know if this influenced the way the study was planned, and conducted or whether the raw data was manipulated [20]. Our finding on the funding source was similar to the findings of Wang et al which showed industry funding for 24% of studies [11]. Funding sources were studied in various research papers to determine the effects of funding on reporting of the outcome. Gaudino et al reported that commercial sponsorship was associated with a significantly greater likelihood of favourable outcomes reported in invasive cardiovascular interventions [21]. In studies on orthopaedic surgery, the association between industry funding and favourable outcomes was found to be significant, which is in agreement with our results [22]. Bekelman et al found that industry funding greatly increased the chances of pro-industry or positive reporting of results, with an odds ratio of 3.6 [23]. Ramagopalan et al analysed the studies registered on https://clinicaltrials.gov/ and found that the industry funding was associated with statistically significant outcomes [24]. During the Covid-19 pandemic, the funding from all fronts was moved towards Covid-19 research, whereas research into viruses before the pandemic comprised only 2%, which went up to 10-20% [25]. There has been a rapid churning out of papers and the peer review process cannot keep pace with this explosion in publications on Covid-19 [26]. Also, some major retractions have been seen from even prestigious journals [27]. Thus, there is a greater need to study the association between funding and outcomes, as apart from the hurry to publish, there was an overwhelming volume of research wherein the stringent peer review process took a back seat.
The ethical implications of industry funding are manifold; pharmaceutical companies need to show positive outcomes as it is in their best interest and favours their financial goals. The public at large may view the research outcomes of industry-funded studies favouring their product as untrustworthy [28]. Keiselheim et al found that the practitioners were vigilant regarding the funding source of trials and believed that industry investment in biomedical research influences the rigor of trials [29]. Kesselheim’s study indicated a reduction in willingness to believe in such results, and thus, a low likelihood of translation of the clinical research into clinical practice [29]. The probable reasons for association of industry funding with reporting of positive outcomes could be that the industry usually funds trials with promising molecules, poor-quality studies [30], with a choice of inappropriate comparator (reduced dose of comparator or change in their route) [31] and publication bias which may be due to the reluctance of the industry or editors/reviewers to publish negative results [32].
Our study emphasises the need to conduct studies with higher methodological quality, in light of the finding of an increased number of open-label studies. Efforts must be made to raise awareness regarding the reporting of conflicts of interest. Once identified, they must be managed at the level of the institute or Ethics Committee. Special efforts are required to identify and implement mitigation strategies to reduce the impact of COI on the design, conduct, analysis and interpretation of the studies. There is also a need for industry-funded studies to report studies with negative results.
The study has a few limitations. We have taken only those clinical drug trials having a comparator arm for the study and have not included non-drug interventions or single-arm studies. Also, we have broadly classified the funding sources as industry and non-industry funded without taking into account other funding sources.
We conclude that reporting of statistically significant outcomes was more likely for trials that have reported a conflict of interest of at least one author versus those studies that have not. Also, industry funded studies are more likely to report statistically significant outcomes.
Conflict of Interest and funding: None to declare.
Data sharing:The authors are willing to share data with other interested researchers.
References
- Fragkou PC, Belhadi D, Peiffer-Smadja N, Moschopoulos CD, Lescure FX, Janocha H, et al. Review of trials currently testing treatment and prevention of COVID-19. Clin Microbiol Infect. 2020;26(8):988–98. https://doi.org/10.1016/j.cmi.2020.05.019
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard With Vaccination Data. World Health Organization. 2021 [Cited 2023 March 08]. p. 1–5. Available from: https://covid19.who.int/%0Ahttps://covid19.who.int/%0Ahttps://covid19.who.int/region/searo/country/bd
- Treatment and vaccines for Covid 19. [Cited 2022 Dec 21]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines-covid-19
- WHO Coronavirus (COVID-19) Dashboard. [Cited 2022 Dec 21] Available from: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization-archived-information
- Yaphe J, Edman R, Knishkowy B HJ. The association between funding by commercial interests and study outcome in randomized controlled drug trials. Family practice; 2001. p. 565–8. https://doi.org/10.1093/fampra/18.6.565
- Diels J, Cunha M, Manaia C, Sabugosa-Madeira B, Silva M. Association of financial or professional conflict of interest to research outcomes on health risks or nutritional assessment studies of genetically modified products. Food Policy. 2011;36(2):197–203. https://doi.org/10.1016/j.foodpol.2010.11.016
- Perlis RH, Perlis CS, Wu Y, Hwang C, Joseph M, Nierenberg AA. Industry sponsorship and financial conflict of interest in the reporting of clinical trials in psychiatry. Am J Psychiatry. 2005;162(10):1957–60. https://doi.org/10.1176/appi.ajp.162.10.1957
- Jung RG, Di Santo P, Clifford C, Prosperi-Porta G, Skanes S, Hung A, et al. Methodological quality of COVID-19 clinical research. Nat Commun. 2021;12(1):1–10. https://doi.org/10.1038/s41467-021-21220-5
- Dinis-Oliveira RJ. COVID-19 research: pandemic ver-sus “paperdemic”, integrity, values and risks of the “speed science”. Forensic Sci Res. 2020;5:174–187. https://doi.org/10.1080/20961790.2020.1767754
- Karlsen APH, Wiberg S, Laigaard J, Pedersen C, Rokamp KZ, Mathiesen O. A systematic review of trial registry entries for randomized clinical trials investigating COVID-19 medical prevention and treatment. PLoS One. 2020;15(8 August):1–13. https://doi.org/10.1371/journal.pone.0237903
- Wang Y, Zhou Q, Xu M, Kang J, Chen Y. Characteristics of Clinical Trials relating to COVID-19 registered at ClinicalTrials.gov. J Clin Pharm Ther. 2020;45(6):1357–62. https://doi.org/10.1111/jcpt.13222
- Mehta HB, Ehrhardt S, Moore TJ, Segal JB, Alexander GC. Characteristics of registered clinical trials assessing treatments for COVID-19: A cross-sectional analysis. BMJ Open. 2020;10(6):1–9. https://doi.org/10.1136/bmjopen-2020-039978
- Irwin RS. The Role of Conflict of Interest in reporting of sceintific information. Chest. 2009;136(1):253–9. https://doi.org/10.1378/chest.09-0890
- Yu Y, Shi Q, Zheng P, Gao L, Li H, Tao P, et al. Assessment of the quality of systematic reviews on COVID-19: A comparative study of previous coronavirus outbreaks. J Med Virol. 2020;92(7):883–90. https://doi.org/10.1002/jmv.25901
- Chirico F, Bragazzi N. Declaration of conflict of interest for reviewers in time of COVID-19 should be mandatory. Perspect Clin Res. 2021;12(1):60–1. https://doi.org/10.4103/picr.PICR_363_20
- Sharma S. Disclosure of conflict of interest in scientific publications. Perspect Clin Res. 2020;11(4):137–8. https://doi.org/10.4103/picr.PICR_287_20
- Amiri AR, Kanesalingam K, Cro S, Casey ATH. Does source of funding and conflict of interest influence the outcome and quality of spinal research? Spine J. 2014;14(2):308–14. https://doi.org/10.1016/j.spinee.2013.10.047
- Feldman AM, Mann DL. Restoring public trust in scientific research by reducing conflicts of interest. J Clin Invest. 2019 Aug 26;129(10):3971–3. https://doi.org/10.1172/JCI131448
- Cappola AR, FitzGerald GA. Confluence, not conflict of interest: Name change necessary. JAMA. 2015;314(17):1791–2. https://doi.org/10.1001/jama.2015.12020
- Capps B. Can a good tree bring forth evil fruit? the funding of medical research by industry. Br Med Bull. 2016;118(1):5–15. https://doi.org/10.1093/bmb/ldw014
- Gaudino M, Hameed I, Rahouma M, Khan FM, Tam DY, Biondi-Zoccai G, et al. Characteristics of Contemporary Randomized Clinical Trials and Their Association with the Trial Funding Source in Invasive Cardiovascular Interventions. JAMA Intern Med. 2020;180(7):993–1001. https://doi.org/10.1001/jamainternmed.2020.1670
- Khan SN, Mermer MJ, Myers E, Sandhu HS. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. Am J Orthop (Belle Mead NJ). 2008;37(12):205–12.
- Bekelman JE, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289(4):454–65. https://doi.org/10.1001/jama.289.4.454
- Ramagopalan S V., Skingsley AP, Handunnetthi L, Magnus D, Klingel M, Pakpoor J, et al. Funding source and primary outcome changes in clinical trials registered on ClinicalTrials.gov are associated with the reporting of a statistically significant primary outcome: A cross-sectional study. F1000Research. 2015;4:1–7. https://doi.org/10.12688/f1000research.6312.2
- NIH. Estimates of funding for various research, condition A, (RCDC) disease categories. NCH review. [Cited 2022 Jan 30]. Available from: https://report.nih.gov/funding/categorical-spending#/a.
- Harper L, Kalfa N, Beckers GMA, Kaefer M. The impact of COVID-19 on research. J Pediatr Urol. 2020;16(5):715–6. https://doi.org/10.1016/j.jpurol.2020.07.002
- Mehra MR, Desai SS, Ruschitzka F PA. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19_ a multinational registry analysis. [Cited 2022 Jan 30]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7255293/.
- Kessel M. Restoring the pharmaceutical industry ’ s reputation. Nat Biotechnol. 2014;32(10):983–90. https://doi.org/10.1038/nbt.3036
- Kesselheim AS, Robertson CT, Myers JA, Rose SL, Gillet V, Ross KM, et al. A Randomized Study of How Physicians Interpret Research Funding Disclosures. N Engl J Med. 2012;367(12):1119–27. https://doi.org/10.1056/NEJMsa1202397
- Bero LA, Rennie D. Influences on the Quality of Published Drug Studies. Int J Technol Assess Health Care. 1996 Mar 10 [Cited 2022 Jan 30];12(2):209–37. Available from: https://cambridge.org/core/journals/international-journal-of-technology-assessment-in-health-care/article/abs/influences-on-the-quality-of-published-drug-studies/31C03A503094EF4FAFE0367AF509ACD9
- Djulbegovic B, Lacevic M, Cantor A, Fields KK, Bennett CL, Adams JR, et al. The uncertainty principle and industry-sponsored research. Lancet. 2000;356:635–8. https://doi.org/10.1016/S0140-6736(00)02605-2
- https://doi.org/10.1016/S0140-6736(00)02605-2
