COMMENTARY
Applying the non-maleficence principle to basic research in Alzheimer’s disease
Bor Luen Tang
Published online first on March 19, 2025. DOI:10.20529/IJME.2025.024Abstract
Despite the urgency for new leads towards Alzheimer’s disease (AD) interventions, the impact of such basic research on patient welfare and potential socioeconomic repercussions are considered remote. Nonetheless, basic science research in AD must adhere to the highest level of ethical stringency. Even preliminary advances in AD basic research offer hope that percolates along the line from researchers to patients. A promising basic research result that is subsequently proven unreliable due to irreproducibility or research misconduct would not only dash hopes but might also misdirect downstream efforts. Furthermore, such misadventures could quash promising research directions that, if otherwise carefully and meticulously interrogated, could yield useful leads. Stringency and reproducibility in biomedical research should thus be framed in accordance with the principle of non-maleficence, which I posit should take priority over loose attempts at beneficence that offer more hype than hope.
Keywords: Alzheimer’s disease, beneficence, hype, non-maleficence, research ethics
Introduction
Alzheimer’s disease (AD), the manifestations of which range from progressive mild cognitive impairment to severe cognitive decline [1], underlies 60-70% of age-associated dementia [2]. In the United States (US) alone, an estimated 6.5 million Americans aged 65 or older suffer from AD, and this number is projected to grow to 13.8 million by 2060 [3]. A 2017 meta-analysis estimates the prevalence of AD in Europe at staggering 5.05% [4]. The total cost for healthcare, long-term care and hospice services for people aged 65 and older with dementia in the US is estimated to be $321 billion in 2022, while unpaid caregiving was valued at $271.6 billion in 2021 [3, 6]. The hugely debilitating disease symptoms and heavy socioeconomic burden of AD have prompted extensive research efforts in finding and testing preventive measures and interventions against disease progression.
However, AD has proven to be a complex and difficult disease to tackle [6], and for many years therapeutics have been limited to drugs that provide only temporary relief of cognitive symptoms. These include those that sustain cholinergic activity (the acetylcholinesterase inhibitors donepezil, galantamine and rivastigmine) and a N-methyl-D-aspartate (NMDA) receptor antagonist memantine, none of which alter disease progression. More recently, an oligosaccharide from marine algae, sodium oligomannate (marketed as GV-971, Green Valley Pharmaceuticals), was approved for mild to moderate AD in China [7]. The US Food and Drug Administration (FDA) has granted accelerated approval to two human monoclonal antibodies (mAbs) targeting amyloid-β (or Aβ, which is a key pathological feature in AD), Aducanumab [8] and Lecanemab [9]. The latter mAbs are purported to be disease modifying through the reduction of amyloid load in the brain. At least for Lecanemab, a moderate suppression in measures of cognitive decline was also demonstrated in clinical trials [10, 11], and the mAb (marketed as Leqembi) has recently been given full approval.
Most controversies in AD research have focused on the latter part of the research pipeline, namely clinical trials and the drug testing/approval process in human participants [12, 13]. Here, I focus on more upstream activities associated with AD research pipelines and discuss the importance of stringency and reproducibility at the earlier stages of research that include disease mechanism elucidation and drug target discovery. Given that there is a pressing need to find effective interventions, a perception of urgency in discovering new drugs or therapeutic approaches, based on a beneficence-conferring mind set, would be common. In other words, the more leads that are made available, the better. However, basic science research in AD, as indeed in all research, needs to be conducted with the highest level of stringency, and with full commitment to ensure reproducibility of the science.
The ethics of research in medicine is usually governed by general rules of research compliance and integrity [14, 15], which entail principles such as honesty and accountability. On the other hand, the ethical practice of medicine, including clinical and therapeutic interventions, is guided by established principles of biomedical ethics, namely beneficence, non-maleficence, justice and autonomy [16]. The latter principles are applicable to aspects of clinical research involving human participants, but not usually considered in any depth as far as basic science research is concerned. Here, I argue that stringency and reproducibility in biomedical research should be framed primarily in accordance with the principle of non-maleficence, and that strict non-maleficence must take priority over loosely framed beneficence for a research field in which hype and hope are often indistinguishable until the human trials stage.
Issues and uncertainties in AD clinical research
Dementia research in general and the AD field in particular raise a spectrum of ethical issues and challenges [13]. Some of these are common to clinical research in other neurodegenerative diseases involving cohorts of vulnerable individuals with difficulties in obtaining informed consent, equipoise and justice in clinical trials [12], and ethical issues in revealing biomarker assessment results to participants [17]. Questions have been asked as to whether the rather modest reduction in cognitive decline associated with humanised antibodies was clinically meaningful [18, 19], and whether the marginal benefits were worth the risk of neurovascular damage clearly manifested by a small fraction of the trial participants [20, 21, 22, 23].
The lack of success in the development of effective therapies in AD [6] has prompted a shift towards earlier diagnosis [24] or detection of AD, or a state of mild cognitive impairment or prodromal AD, and efforts to improve brain health outcomes and help maintain affected individuals to remain symptom-free [25]. However, this move towards early detection and prevention of AD also presents ethical issues due to uncertainties in biomarker-based predictions [26, 27]. Furthermore, although many dietary supplements, nutraceuticals, phytochemicals and repurposed drugs have conferred protection against AD in animal experiments, there is no definitive evidence for the efficacy for any of these from large trials and meta-analysis of trials in humans [28, 29, 30, 31, 32].
Controversies and misconduct in AD basic science research
The field of neurodegenerative disease research has a notably high number of retractions, as shown by searches of Scopus (https://www.scopus.com) performed with the term “retraction” within the returns from several search terms corresponding to the most prevalent of human diseases, namely “stroke”, “cancer”, “diabetes” and “COVID-19”, up to the year 2022. When expressed per thousand (‰), these have values ranging from 1.88 to 2.76 (see Figure 1). On the other hand, a similar search of “retraction” within the returns from search terms of the four major neurodegenerative diseases, namely AD, Parkinson’s, Amyotrophic Lateral Sclerosis and Huntington’s disease have values ranging from 3.61 to 5.23. These results suggest that within the broad field of biomedicine, neurodegenerative disease research has higher than average rates of errors or misconduct.
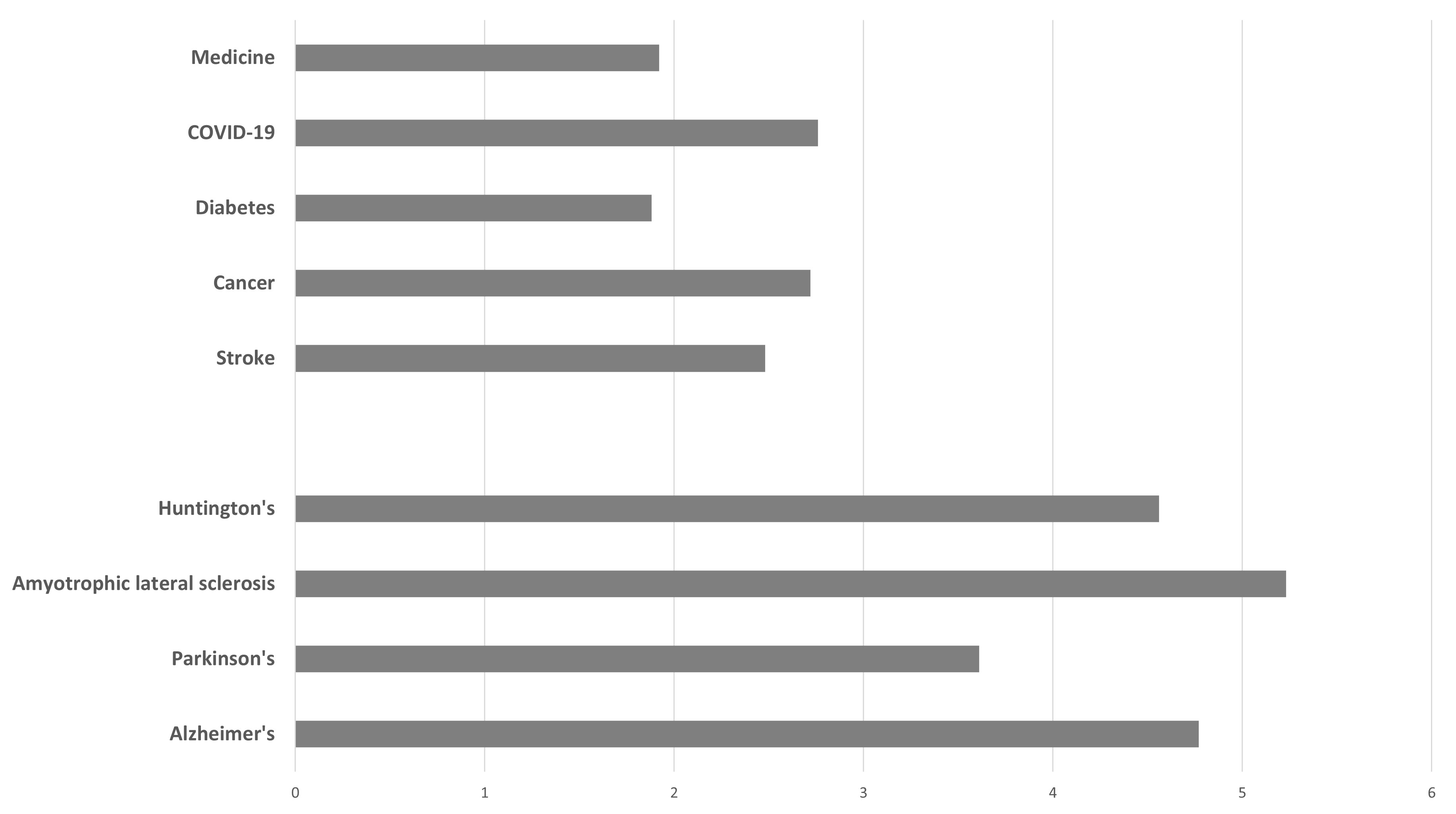
Figure 1. A graphical comparison of retraction index (‰) obtained from searches of Scopus (https://www.scopus.com/sources) with the term “retraction” occurring within the returns of searches with the term “medicine” and major human diseases “stroke”, “cancer”, “diabetes” and “COVID-19”, as well as the four major neurodegenerative diseases.
AD research in particular is not short of important studies that were subsequently retracted because of acts of misconduct [33, 34] . More recently, three new emerging cases have amassed wide attention and discussion. These are briefly reviewed below.
Case 1 concerns the work of Hoau-Yan Wang and his collaborators in Cassava Sciences, who found that soluble Aβ42 peptides could change the conformation of a cytoskeletal protein Filamin A, thus promoting the latter’s interaction with neuronal receptors in enhancing toxic signaling of soluble amyloid. This neurotoxic signalling could contribute to neuronal loss in AD. The researchers reported in two papers that a compound, PTI-125 or Simufilam [35], restores Filamin A’s native conformation with the receptor and reduces AD pathologies in mouse models. Cassava Science has promptly proceeded to conduct phase II and larger phase III clinical trials with Simufilam. However, image and data irregularities were found in these papers [36, 37]. Wang has now had seven papers, including two earlier papers on Filamin A, retracted. A committee convened by the City University of New York found evidence of scientific misconduct involving 20 research papers, including the papers above [36]. There are also potential issues with the clinical trials of Simufilam.
In Case 2, a highly cited discovery of a unique 56 kDa soluble amyloid beta assembly (Aβ*56), published by Sylvain Lesné and colleagues in Nature in 2006, is now severely marred by allegations of image manipulation identified in the paper [38, 39]. The discovery of Aβ*56 and illustration of its memory-disrupting activity in animals has contributed to the notion of soluble Aβ oligomers being the actual toxic entities in AD brain [40]. Similar issues with western blot image manipulations have been identified in a number of Lesné’s subsequent papers and at least one paper directly concerning Aβ*56 has attracted an editorial expression of concern [41]. Karen Ashe, the senior author on the 2006 paper in Nature, has maintained that soluble type 1 Aβ oligomers, such as Aβ*56, remain a valid AD target [42]. Some prominent researchers in the field have also downplayed the impact of Aβ*56 on the field and the setback associated with the allegations of misconduct [43], but it remains possible that some thoughts and efforts in target-based AD drug development might already have been somewhat misleading[44].
Case 3 concerns a (now retracted) paper in Nature in 2009 by Marc Tessier-Lavigne’s group (then in Genentech), in which it was reported that β-secretase-shed ectodomain of the Amyloid Precursor Protein’s (N-APP) interacts with Death Receptor 6 (DR6), and that their interaction activates a widespread caspase-dependent cell death programme that would contribute to AD pathology [45]. Doubts have since arisen on the validity of the paper’s findings, and integrity of data (including multiple comments in PubPeer). Genentech’s internal enquiry has indicated potential data falsification in the paper which ended all subsequent pursuits of drug development based on the finding [46]. A report released by Genentech on its investigations had nonetheless concluded that there was no evidence of fraud or intentional wrongdoing in the work [47, 48], and neither did a subsequent investigation report by Stanford specifically point to research misconduct associated with the Nature paper. Despite issues with the validity of the paper’s main conclusions [49], there were no apparent attempts on the part of the authors to voluntarily correct or retract the 2009 Nature paper [50], and retraction was only subsequently done after Stanford had completed its investigations on Tessier-Lavigne.
The controversies and cases of research misconduct described above thus expose certain shortcomings in basic science research on AD. Driven by a competitive culture and the need to excel, some researchers (and perhaps their institutions and companies) seem overzealous about hyping the significance of their findings, ignoring details, anomalies and evidence that runs counter to their narrative. As a result, the painstaking task of ensuring reproducibility, particularly in varying experimental contexts to affirm the broad validity of findings and conclusions, is compromised. There is added pressure on basic science to extend and rush knowledge generation in translation to the clinic.
The importance of stringency and reproducibility in AD basic science research: Hype and harm
The cases of research misconduct in upstream basic science research in AD are alarming and damaging for several reasons. The first of these is that basic research findings of translation potential generally receive more media (both scientific and public) attention, which stimulates hope for patients and caregivers, as well as those associated with them. While institutional and industrial/commercial entities look towards the generation of patents and profits, fellow researchers join in to follow up on published results to replicate, refine approaches and seek related alternatives.
However, as hope percolates along the research pipeline from researchers to the other parties both with and without vested interests, it could be completely dashed by knowledge of research irreproducibility or misconduct associated with the original finding. For example, Genentech’s investigation report on the 2009 paper in Nature shared the following sentiment [47]:
Genentech’s termination of the DR6 drug discovery program marked the end of many years of challenging and often frustrating research that many hoped would culminate in a treatment for Alzheimer’s Disease. Many scientists who worked on the project were disheartened by having devoted substantial time and energy to a program whose underlying biology was ultimately proven wrong. That sentiment gave rise to rumors about why the DR6 program failed.
One could imagine the disappointment and distress of patients and trial participants when they learn that the basic science results underlying Simufilam is questionable. In this regard, there appears to be a misalignment between the researchers and the supposed beneficiaries of the research. In other words, those researchers whose only goal is to make findings and push these towards translation would be in conflict with the true interest of patience and caregivers, which would be in obtaining reliable and efficacious new therapeutics.
The path between primary basic science finding and clinical application is long and involves considerable efforts and investments in research experimentation and development. The original amyloid cascade hypothesis [51] or its alternatively phrased variants [52, 53] have been critical in driving rationale AD drug development, particularly those involving the development of humanised antibodies against various forms of Aβ. As mentioned above, reports on the discovery of Aβ*56 have also added impetus to this direction. However, the amyloid-targeting immunotherapy approach is belied by multiple failures at phase III trials [54, 55]. AD is a complex disease and there are many reasons why amyloid lowering observed in diseased participants in several trials did not quite translate into significant reduction in cognitive decline as hoped [54, 56]. What the field does not need is a false lead based on fraudulent research that could waste follow-up efforts and resources, or worse, that could undermine progress in an otherwise rational and logical direction.
A promising basic science research result that is subsequently shown to be unreliable might also quash research directions that, if otherwise thoroughly and carefully interrogated, could yield useful knowledge or leads. FLNA and Aβ*56, for example, should remain interesting subjects of study in their own right and might still be valid targets for AD drug development. However, the fear would be that the negative press associated with the controversies and documented misconduct might deter other researchers from following their investigations and might also dampen the confidence of funders to support research along these lines. Careless or fraudulent work could therefore effectively kill not just ongoing projects, but also entire research leads.
Taking a well-known historical example from the biomedical sciences, the Hwang human stem cells scandal, involved fraudulent claims on successful cloning of human embryonic stem cells by somatic cell nuclear transfer [57]. The Hwang scandal affected the entire biotechnology enterprise [58], and the fallout from the Hwang case is felt globally by all researchers and programmes on stem cells [59]. Along similar lines, the fraud associated with work on adult cardiac stem cells and heart regeneration from Piero Anversa’s group [60] have affected several planned clinical trials [61], while misconduct [62] and deaths of transplant recipients in the Paolo Macchiarini case [63] have hurt clinical research in the respective fields. The loss of public trust in a particular field of scientific research resulting from fraud and misconduct is therefore clear.
Finally, careless or fraudulent work could harm human trial participants and patients directly. It is conceivable that AD therapeutic leads derived from fraudulent works that progress to clinical trials could pose unwarranted risks to human lives and health. Such risks could in fact be masked by the advanced age and poor health of participants and patients, contributing to significant attrition during trials. Although there are stringent measures in place to ensure safety and continuous monitoring of adverse effects in trials, risks generated by upstream research should not be allowed to stealthily creep into human experiments. Thus, although the above research misconduct cases in AD research represent a small minority, the repercussions of these cases could be disproportionately large.
It should also be noted that even for basic research results that are valid and reproducible, the potential harm of overselling and overhyping these results or findings remain. Many AD therapeutic leads, however promising in animal experiments and other preclinical assessments, have failed miserably in human trials. There is a long list of such examples that would serve as a reminder against overhyping any AD leads [64].
The non-maleficence principle and its application to AD basic science research
One of the approaches to biomedical ethics is Principlism, with its conceptual doctrines spelt out in the definitive framework presented in Beauchamp and Childress’ classic text [16]. The four principles of biomedical ethics (beneficence, non-maleficence, justice and autonomy) as they are framed are very much physician-patient centred and are thus more commonly applied in the practice of medicine, including therapeutic and healthcare activities. These principles are also critically important for clinical research directly involving human subjects, in which the need for careful risk-benefit analyses, the establishment of informed consent and various regulatory constraints are in place to minimise harm and to protect the interest of subjects and participants.
As basic science research usually involves disease-simulating cell/tissue and animal models rather than human subjects, the four principles of biomedical ethics are not usually given priority, if at all. However, because basic research results would ultimately impact human subjects, I would argue that these principles are worthy of consideration and application even at the level of basic research.
Riding on the hope of many in overcoming a devastating disease with complex etiology and no effective therapy despite decades of research, the AD research enterprises might tend towards adopting a conceptual attitude of “the more the merrier” in terms of new leads and findings. It could be argued that such an attitude might appear to be somewhat in line with the biomedical ethics principle of conferring all round beneficence to those at the receiving end, ie the patients, caregivers, physicians, as well as the researchers themselves (the latter in terms of obtaining research funding, publications and patents that are important for career advancement). However, such a mindset might also contribute to compromises in stringency in validation of findings and replication efforts to ensure reproducibility, or a tendency to overhype any promising findings or leads. The latter would result in wasteful downstream efforts in attempts to translate the research, loss of hope in a particular research direction or lead, and in the worst case, bring harm to trial participants or patients.
One notable trend in contemporary basic academic research is the eagerness on the part of researchers and institutions to commercialise research results or inventions. The commodification of research is a broad and complex topic. However, as pointed out by Hans Radder, there is a likelihood that “…researchers who apply for a patent, that is, an economic or financial monopoly, accept assessment criteria that are much weaker than the usual epistemological standards” [65, 66]. One might argue that this need not be so, and that good and honest science can be done even when it is nested within a for-profit mindset. However, when one considers the classical norms of science as formulated by Robert Merton, ie those of universalism, communalism, disinterestedness and organised skepticism [67], it becomes clear that one who does research for profit (or prioritises monetary rather than knowledge gains) would find these norms difficult to embrace. If nothing else, the findings and results are no longer communal, and “disinterestedness” would be unintuitive and likely untenable.
It would thus be pertinent for those in AD basic science research to adopt a mindset that is more in line with the aphorism of primum non nocere, or “first, do no harm”, which summarises the guiding principle of non-maleficence in clinical practice and research. An ethical stance of strict non-maleficence would be more prudent than a loose or distorted perception of beneficence. Such a non-maleficence mindset should ideally also be adopted by all other stakeholders associated with the discovery, reporting and news dissemination of upstream basic research in AD, from researchers/authors to reviewers/editors/journals, research institutions/companies, as well as the public media. The economisation of science and research that occurs with neoliberalism [68] has likely also promoted the elaboration hype in science communication [69]. All stakeholders should pay heed to the cultivation of humility and reservations in the reporting and public communication of scientific findings. In applying the non-maleficence principle, the relevant parties should exercise prudence and make only cautious and accurate statements in updating and advising downstream stakeholders and the public. They must not allow the lure of potential translational benefits and financial returns to instil any hype in the reporting of research findings or results.
Epilogue
The call above is for an emphasis on stringency and reproducibility in basic science research that would minimise hype and follow the biomedical ethics principle of non-maleficence. There is, first and foremost, a need to uphold truthfulness in research, particularly so for complex and devastating diseases like AD [70]. The most stringent and reproducible basic science results would logically have the highest chance of being translatable in the clinic, thus going further towards fulfilling the beneficence principle. Research resources are ultimately provided by taxpayers, a fraction of which are indeed the patients and their caregivers. It is only fair and just for the contribution of the latter, however indirect, to be adequately reciprocated by the researchers by doing their best in providing hope rather than propagating hype. Hype of any type would mislead patients and give rise to false hope, potentially misleading them into making uninformed and erroneous decisions on participating in trials or treatment. Preying on one’s vulnerability with hype would essentially be an infringement of one’s autonomy, albeit based on deception rather than force.
It follows from the above that the practice of stringency and reproducibility in basic science research would be very much in line with all four basic principles of biomedical ethics, i.e., those of non-maleficence, beneficence, justice and autonomy. There are other ethical perspectives that would converge upon the same notion. For example, Alex John London’s conception of research ethics grounded in justice and for the common good [71] would imply that researchers should give proper weight to all downstream stakeholders, who are all part of the collective social undertaking for advances in scientific knowledge. Researchers should therefore bear in mind that patients and their caregivers are the end in themselves rather than the means for their personal success.
Author: Bor Luen Tang ([email protected], https://orcid.org/0000-0002-1925-636X), Department of Biochemistry, Yong Loo Lin School of Medicine, National University Health System, National University of Singapore, SINGAPORE.
Conflict of Interest: None declared Funding: None
Acknowledgments: The author is grateful to both reviewers, whose constructive comments improved the manuscript.
To cite: Tang BL. Applying the non-maleficence principle to basic research in Alzheimer’s disease. Indian J Med Ethics. 2025 Jul-Sep; 10(3) NS: 233-239. DOI: 10.20529/IJME.2025.024
Submission received: March 4, 2024
Submission accepted: December 23, 2024
Published online first: March 19, 2025
Manuscript Editor: Sanjay A Pai
Peer Reviewers: Timothy Daly, Roop Gursahani, and an anonymous reviewer
Copyright and license
©Indian Journal of Medical Ethics 2025: Open Access and Distributed under the Creative Commons license (CC BY-NC-ND 4.0), which permits only noncommercial and non-modified sharing in any medium, provided the original author(s) and source are credited.
References
- Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE,et al. Alzheimer’s disease. Lancet. 2021; 397(10284):1577-1590. https://doi.org/10.1016/S0140-6736(20)32205-4
- World Health Organization, Dementia. Geneva: WHO2023 Mar 15[Cited 2023 Sept 23]. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia
- Alzheimer’s Association. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022; 18(4): 700-789. https://doi.org/10.1002/alz.12638
- Niu H, Álvarez-Álvarez I, Guillén-Grima F, Aguinaga-Ontoso I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017; 32(8): 523-532. https://doi.org/10.1016/j.nrl.2016.02.016
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021; 17(3): 327-406. https://doi.org/10.1002/alz.12328
- Cummings JL, Morstorf, T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther. 2014; 6: 37. https://doi.org/10.1186/alzrt269
- Syed YY. Sodium Oligomannate: First Approval. Drugs, 2020; 80(4):441-444. https://doi.org/10.1007/s40265-020-01268-1
- US Food and Drug Administration. FDA Grants Accelerated Approval for Alzheimer’s Drug.. FDA; 2021 Jun 7[Cited 2023 Sept 23]. Available from: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
- US Food and Drug Administration FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. 2023 Jan 6[Cited 2023 Sept 23]. Available from: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment
- McDade E, Cummings JL, Dhadda S, Swanson CJ, Reyderman L, Kanekiyo M, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res. Ther. 2022; 14(1): 191. https://doi.org/10.1186/s13195-022-01124-2
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al.Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023; 388(1):9-21. https://doi.org/10.1056/NEJMoa2212948
- Davis DS. Ethical issues in Alzheimer’s disease research involving human subjects. J. Med. Ethics 2017; 43(12): 852-856. https://doi.org/10.1136/medethics-2016-103392
- Götzelmann TG, Strech D, Kahrass H. The full spectrum of ethical issues in dementia research: findings of a systematic qualitative review. BMC Med, Ethics 2021; 22(1): 32. https://doi.org/10.1186/s12910-020-00572-5
- Resnik D. Shamoo AE. The Singapore statement on research integrity. Account. Res. 2011; 18(2):71-75. https://doi.org/10.1080/08989621.2011.557296
- The European Code of Conduct for Research Integrity. All European Academies (ALLEA) 2023 Jun [Cited 2024 Feb 2]. Available at https://allea.org/code-of-conduct/
- Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Oxford: Oxford University Press; 2019
- Bunnik EM, Smedinga, M, Milne, R, Georges, J, Richard, E, Schermer MHN. Ethical Frameworks for Disclosure of Alzheimer Disease Biomarkers to Research Participants: Conflicting Norms and a Nuanced Policy. Ethics Human Res. 2022; 44(6): 2-13. https://doi.org/10.1002/eahr.500146
- Knopman DS, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement. 2021; 17(4): 696-701. https://doi.org/10.1002/alz.12213
- Liu KY, Schneider LS, Howard R. The need to show minimum clinically important differences in Alzheimer’s disease trials. Lancet Psychiatry 2021; 8(11): 1013-1016. https://doi.org/10.1016/S2215-0366(21)00197-8
- Lacorte E, Ancidoni A, Zaccaria V, Remoli G, Tariciotti L, Bellomo G, et al. Safety and Efficacy of Monoclonal Antibodies for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Published and Unpublished Clinical Trials. J. Alzheimers Dis. 2022; 87(1): 101-129. https://doi.org/10.3233/JAD-220046
- Salloway S, Chalkias S, Barkhof F, Burkett P, Barakos J, Purcell D, et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol. 2022; 79(1): 13-21. https://doi.org/10.1001/jamaneurol.2021.4161
- The Lancet. Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet 2022; 400(10367) (2022): 1899. https://doi.org/10.1016/S0140-6736(22)02480-1
- Yeo-Teh NSL, Tang BL. A Review of Scientific Ethics Issues Associated with the Recently Approved Drugs for Alzheimer’s Disease. Sci. Eng. Ethics 2023; 29(1):2. https://doi.org/10.1007/s11948-022-00422-0
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014; 13(6): 614-629. https://doi.org/10.1016/S1474-4422(14)70090-0
- Gregory S, Saunders S, Ritchie CW. Science disconnected: the translational gap between basic science, clinical trials, and patient care in Alzheimer’s disease. Lancet Healthy Longev. 2022 3(11): e797-803. https://doi.org/10.1016/S2666-7568(22)00219-7
- Schermer MHN, Richard E. On the reconceptualization of Alzheimer’s disease. Bioethics 2019; 33(1):138-145. https://doi.org/10.1111/bioe.12516
- Daly T. The ethics of revolution in Alzheimer’s research. Lancet Healthy Longev. 2023; 4: e61. https://doi.org/10.1016/S2666-7568(22)00289-6
- Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. Alzheimer’s Disease Cooperative Study. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015; 85(16):1383-91. https://doi.org/10.1212/WNL.0000000000002035
- Burckhardt M, Herke, M, Wustmann, T, Watzke, S, Langer, G, Fink A. Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst. Rev. 2016; 4:CD009002. https://doi.org/10.1002/14651858.CD009002.pub3
- Farina N, Llewellyn D, Isaac MG, Tabet N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2017; 4(4) (2017): CD002854. https://doi.org/10.1002/14651858.CD002854.pub4
- Burckhardt M, Watzke S, Wienke, A, Langer, G, Fink A. Souvenaid for Alzheimer’s disease. Cochrane Database Syst. Rev. 2020; 12:CD011679. https://doi.org/10.1002/14651858.CD011679.pub2
- Desai RJ, Mahesri M, Lee SB, Varma VR, Loeffler T, Schilcher I, et al. No association between initiation of phosphodiesterase-5 inhibitors and risk of incident Alzheimer’s disease and related dementia: results from the Drug Repurposing for Effective Alzheimer’s Medicines study. Brain communications 2022; 4(5): fcac247. https://doi.org/10.1093/braincomms/fcac247
- Oransky I. Former Columbia postdoc faked Alzheimer’s research in Cell and Nature. Retraction Watch 2015 Apr 7[Cited 2023 Sep 23]. Available from: https://retractionwatch.com/2015/04/07/former-columbia-postdoc-faked-alzheimers-research-in-cell-and-nature/
- Alexander R, Peterson CJ, Yang S, Nugent K. Article retraction rates in selected MeSH term categories in PubMed published between 2010 and 2020. Account. Res. 2023; 20:1-14. https://doi.org/10.1080/08989621.2023.2272246
- Simufilam. Alzforum.org. [Cited 2023 Sep 23]. Available from: https://www.alzforum.org/therapeutics/simufilam
- Expression of Concern: Wang et al., “Reducing Amyloid-Related Alzheimer’s Disease Pathogenesis by a Small Molecule Targeting Filamin A”. J. Neurosci. 2022; 42(3): 529. https://doi.org10.1523/JNEUROSCI.2306-21.2021
- Piller C. Co-developer of Cassava’s potential Alzheimer’s drug cited for ‘egregious misconduct’. Science 2023 Oct 12[Cited 2024 Feb 2]. Available from: https://www-science-org.libproxy1.nus.edu.sg/content/article/co-developer-cassava-s-potential-alzheimer-s-drug-cited-egregious-misconduct
- Piller, C. “Blots on a field?” Science 2022 July 21 [Cited 2023 Sept 23] Available from: https://www-science-org.libproxy1.nus.edu.sg/content/article/potential-fabrication-research-images-threatens-key-theory-alzheimers-disease
- Rogers MB. Sylvain Lesné, Who Found Aβ*56, Accused of Image Manipulation. Alzforum.org. 2022 July 22[Cited 2023 Sept 23]. Available from: https://www.alzforum.org/news/community-news/sylvain-lesne-who-found-av56-accused-image-manipulation
- Tolar M, Hey J, Power A, Abushakra S. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer’s Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int J Mol Sci. 2021 22(12):6355. https://doi.org/10.3390/ijms22126355
- Foley JF. Editorial expression of concern. Sci. Signal. 2022; 15(739):eadd4322. https://doi.org/10.1126/scisignal.add4322
- Ashe KH. Alzheimer’s target still viable but untested. Science 2022; 377(6609): 935. https://doi.org/10.1126/science.ade4073
- Selkoe D, Cummings J. News story miscasts Alzheimer’s science. Science 2022; 377(6609):934-935. https://doi.org/10.1126/science.ade1872
- Appenzeller T. Editor’s note. 2022 Aug 25 [Cited 2023 Sep 23]; Available from: https://www-science-org.libproxy1.nus.edu.sg/doi/10.1126/science.ade2733
- Nikolaev A, McLaughlin T, O’Leary DDM, Tessier-Lavigne M. Retraction Note: APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 2024; 625(7993):204. https://doi.org/10.1038/s41586-023-06943-3
- Baker T. Internal review found ‘falsified data’ in Stanford President’s Alzheimer’s research, colleagues allege. Stanford Daily 2023 Feb 17 [Cited 2023 Sept 23]. Available from: https://stanforddaily.com/2023/02/17/internal-review-found-falsified-data-in-stanford-presidents-alzheimers-research-colleagues-allege/
- Findings of 2023 Genentech Review of 2009 Nature Paper and Related Research. Genentech 2023 Apr 6 [Cited 2023 Sep 23]. Available from: https://www.gene.com/download/pdf/Findings-of-2023-Genentech-Review-of-2009-Nature-Paper-and-Related-Research.pdf
- Kaiser J. No evidence that Stanford President Marc Tessier-Lavigne hid fraud at Genentech, company says. Science 2023 Apr 6 [Cited 2023 Sep 23]. Available from: https://www-science-org.libproxy1.nus.edu.sg/content/article/no-evidence-stanford-president-marc-tessier-lavigne-hid-fraud-genentech
- Olsen O, Kallop DY, McLaughlin T, Huntwork-Rodriguez S, Wu Z, Duggan CD,et al. Genetic analysis reveals that amyloid precursor protein and death receptor 6 function in the same pathway to control axonal pruning independent of β-secretase. J. Neurosci. 2014; 34(19): 6438-6447. https://doi.org/10.1523/JNEUROSCI.3522-13.2014
- Report of the Scientific Panel of the Special Committee of the Stanford University Board of Trustees. Stanford University 2023 July 17 [Cited 2024 Feb 2]. Available from: https://boardoftrustees.stanford.edu/wp-content/uploads/sites/5/2023/07/Scientific-Panel-Final-Report.pdf
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992; 256(5054): 184-185. https://doi.org/10.1126/science.1566067
- Hayden EY, Teplow TB. Amyloid β-protein oligomers and Alzheimer’s disease. Alzheimers Res. Ther. 2013; 5(6): 60. https://doi.org/10.1186/alzrt226
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016; 8(6):595-608. https://doi.org/10.15252/emmm.201606210
- Lozupone M, Solfrizzi V, D’Urso F, Di Gioia I, Sardone R, Dibello V, et al. Anti-amyloid-β protein agents for the treatment of Alzheimer’s disease: an update on emerging drugs. Expert Opin. Emerg. Drugs 2020; 25(3): 319-335. https://doi.org/10.1080/14728214.2020.1808621
- Daly T, Herrup K, Espay AJ. An Ethical Argument for Ending Human Trials of Amyloid-Lowering Therapies in Alzheimer’s Disease. AJOB Neurosci. 2022; https://doi.org/10.1080/21507740.2022.2129858
- Doig AJ, Del Castillo-Frias MP, Berthoumieu O, Tarus B, Nasica-Labouze J, Sterpone F, et al. Why Is research on amyloid-β failing to give new drugs for Alzheimer’s Disease?” ACS Chem. Neurosci. 2017; 8(7): 1435-1437. https://doi.org/10.1021/acschemneuro.7b00188
- Hong S. The Hwang Scandal That “Shook the World of Science”. East Asian Sci. Technol. Soc. 2008; 2(1): 1-7. https://doi.org/10.1215/s12280-008-9041-x
- Fuyuno I. Hwang scandal hits Korean biotech hard. Nature 2006; 439(7074): 265. https://doi.org/10.1038/439265a
- Check E, Cyranoski, D. Korean scandal will have global fallout. Nature 2005; 438: 1056-1057. https://doi.org/10.1038/4381056a
- 60. Ozkan J. Piero Anversa and cardiomyocyte regeneration. Eur. Heart J. 2019; 40(13): 1036-1037. https://doi.org/10.1093/eurheartj/ehz096
- Reardon S. US government halts heart stem-cell study. Nature 2018 Oct 29[Cited 2023 Sept 23]. Available from: https://www-nature-com.libproxy1.nus.edu.sg/articles/d41586-018-07232-0
- Hawkes N. Macchiarini case: seven researchers are guilty of scientific misconduct, rules Karolinska’s president.” BMJ 2018; 361: k2816. https://doi.org/10.1136/bmj.k2816
- Vogel G. Disgraced Italian surgeon convicted of criminal harm to stem cell patient. Science 2022 Jun 16[Cited 2023 Sept 23]. Available from: https://www.science.org/content/article/disgraced-italian-surgeon-convicted-of-criminal-harm-to-stem-cell-patient
- Asher S, Priefer R. Alzheimer’s disease failed clinical trials. Life Sci. 2022; 306:120861. https://doi.org/10.1016/j.lfs.2022.120861
- Radder H. How (not) to be held accountable in research: The case of the Dutch integrity code.” Accountability in Research 2023; 30(5): 261-275. https://doi.org/10.1080/08989621.2022.2115888
- Radder H. How (not) to be held accountable in research: A reply to my critics. Accountability in Research 2023; 30(5):284-291. https://doi.org/10.1080/08989621.2023.2193694
- Merton, RK. The Sociology of Science: Theoretical and Empirical Investigations. Chicago: University of Chicago Press; 1973.
- Davi H, Modicom, PY, Durand, JK, Eldin C. How has neoliberalism weakened science? Nat. Sci. Soc. 2021; 29(3): 356-59. https://doi.org/10.1051/nss/2021053
- Soto-Sanfiel MT, Chong CW, Latorre JI. Hype in Science Communication: Exploring Scientists’ Attitudes and Practices in Quantum Physics. arXiv 2023 Nov 13[Cited 2024 Feb 2]. Available from: https://arxiv.org/abs/2311.07160
- Daly TP. Need for truthfulness in dementia research. British Medical Journal 2023; 380:255.
- London, AJ. For the Common Good. Oxford: Oxford University Press; 2021
