DISCUSSION
Vioxx redux – or how I learned to worry about industry-sponsored clinical trials
James M Brophy
DOI: https://doi.org/10.20529/IJME.2016.065
Published online: August 30, 2016
I read with interest Mark Wilson’s recent article, “The New England Journal of Medicine: commercial conflict of interest and revisiting the Vioxx scandal” (1). I believe this is an important contribution that underlines the aphorism “Those who don’t know history are doomed to repeat it.” As Vioxx is a seminal example, it is important to place it in its proper context, examining if this malfeasance extends beyond the VIGOR study (2). While the epicentre of this conflict of interest surely begins with the sponsor, I believe the following essay demonstrates that this wave of egregiously unethical behaviour can exist and be propagated only with the complicity of academic investigators, medical journals, a flawed peer-review system and an uncritical medical readership. Perhaps the most troubling is that the factors that coalesced into the Vioxx scandal are, if anything, more ubiquitous today, mandating increased vigilance to decrease the probability of “getting fooled” again.
As detailed by Wilson (1), there were discrepancies between what the sponsor reported to the FDA and what was included in the NEJM article. How to interpret these discrepancies? Was it merely an isolated, honest oversight by the sponsor or was it reflective of a systematic attempt to downgrade the risk of rofecoxib (Vioxx®)? An in-depth examination of other peer-reviewed articles on rofecoxib may help answer this question and provide general insights into the reliability or “safety” of medical publishing.
In the paradigm of evidence-based medicine, meta-analyses have been elevated to the top of the evidential pyramid. Consequently, the publication of a meta-analysis of all the randomised trials available on rofecoxib in the leading cardiovascular subspecialty journal (3) on October 15, 2001, may have been projected to, and undoubtedly did, reassure practitioners, the public, and regulators about the “unexpected” cardiovascular safety concerns raised by the VIGOR study. This “timely” reassurance was most likely prompted, or at least partially prompted, by a high-profile JAMA publication (4) on August 22, 2001, which raised “a cautionary flag about the risk of cardiovascular events with COX-2 inhibitors”. A detailed examination of this meta-analysis sheds considerable light on this scandal and raises further questions about the independence of journal editors and the competency of the peer-review system.
The meta-analysis (3), published by seven authors (five Merck employees and two academic consultants to Merck), provided data from 23 completed trials involving 28,000 patients with osteoarthritis, rheumatoid arthritis, Alzheimer’s disease, or lower back pain, totalling 14,000 patient years at risk. The authors conclude: “This analysis provides no evidence for an excess of cardiovascular events for rofecoxib relative to either placebo or the non-naproxen non-steroidal non-inflammatory drugs (NSAIDs) that were studied. Differences observed between rofecoxib or naproxen are likely the result of the antiplatelet effects of the latter agent.” The potential completeness of the data sources from the sponsor files and the use of individual patient-level data were the apparent strengths of this study.
However, enthusiasm for this publication should have been tempered by the lack of clarity on the adjudication process (see VIGOR above and ADVANTAGE below), and the fact that only one of the 23 trials included had been peer-reviewed and published before this meta-analysis was published. Moreover, despite the reassuring conclusions, the data actually tell a different story. The relative risk for the combined cardiovascular endpoint was 0.84 (95% CI 0.51 to 1.38), 0.79 (95% CI 0.40 to 1.55) and 1.69 (95% CI 1.07 to 2.69) when comparing rofecoxib to placebo, non-naproxen NSAIDS and naproxen, respectively. Given that these data are, therefore, compatible with a possible 38% or 55% increase or even a 49%–60% decrease in cardiovascular outcomes compared to placebo or non-naproxen NSAIDs, and given that differences of that magnitude would surely be considered clinically significant, the proper interpretation is that these data are insufficient, or underpowered, to conclude whether rofecoxib is safer, equal to or more dangerous than these comparators. Lest the statistically uninformed reader feel that these are difficult, obtuse concepts and that once again, only honest mistakes have occurred, an internal Merck email pre-publication review of this article is revealing. The email, from a Merck scientist, stated: “The second line of the discussion says, ‘There was no evidence that rofecoxib was associated with excess CV events compared with either placebo or non-naproxen NSAIDs’ – that seems wishful thinking, not a critical interpretation of the data.”1
With regard to the naproxen versus rofecoxib comparison, the meta-analysis showed an unequivocal clinically and statistically significant increase in risk. The authors explained this difference not by suggesting that rofecoxib posed any danger, but by postulating that naproxen had cardioprotective effects. The apocryphal cardioprotective benefit of naproxen was a recurrent theme in the minimisation of rofecoxib risks. In point of fact, there was no published evidence to support this opinion. For example, a PubMed search up to the date that VIGOR was published was unable to identify a single reference to naproxen and myocardial infarction (MI) (see Figure 1). The genesis of the hypothesis on the clinical cardioprotective effects of naproxen first advanced in the NEJM VIGOR publication was based on a reference to the benefits observed in a small clinical European study, not of rheumatology patients, but of acute coronary patients and not even with naproxen as a treatment option, but rather, with a completely different NSAID, flurbiprofen, as the treatment option (5)!
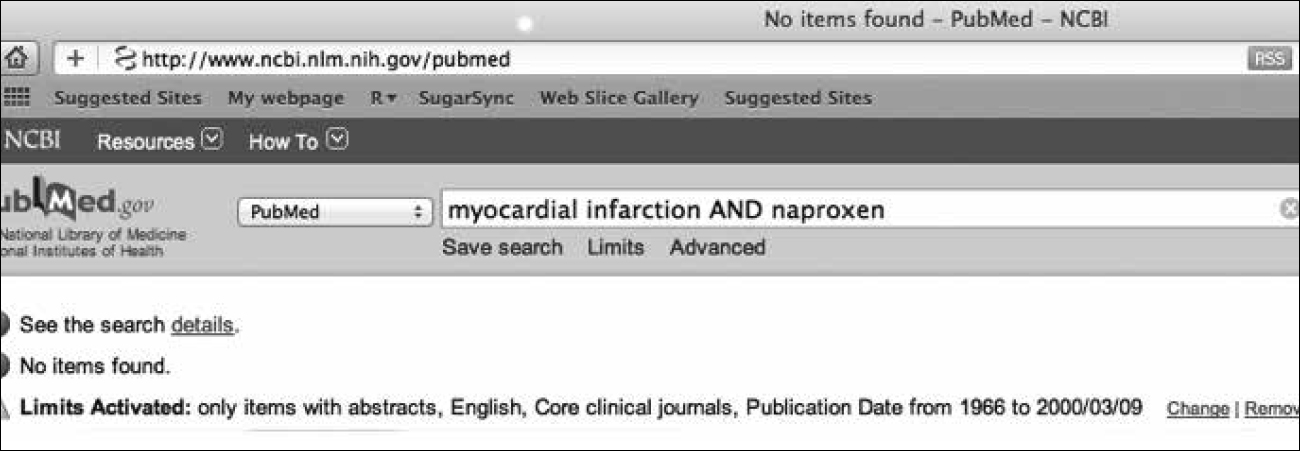
Further, the complicity of the peer-review process that permitted the circulation of these untruths is concretely and blatantly in evidence if one examines the submission, acceptance and publication dates of the meta-analysis. The publication record (printed on the first page of the article) shows that it was received by the journal on October 2, 2001, accepted for publication on October 3, 2001, and electronically published on October 15, 2001. I would challenge this journal, or any other journal, to provide other examples of scientific articles undergoing quality peer and editorial review in 24 hours. The integrity and quality of the scientific review process appears to have been completely compromised by the expediency of the journal’s editors to respect the sponsor’s urgent need for quick publication to counter the cautionary JAMA article.
Consider next the publication of the randomised ADVANTAGE study in Annals of Internal Medicine on October 7, 2003 (6). The objective of the study was ostensibly to assess the tolerability of rofecoxib compared with naproxen for the treatment of osteoarthritis in 5557 patients. The authors reported no statistically significant difference in terms of the occurrence of MI (“Five myocardial infarctions occurred in the rofecoxib group, and 1 occurred in the naproxen group (p>0.2).”) A later correction (7) reported 6 MIs with rofecoxib versus 1 with naproxen. According to data released by the FDA, eight rofecoxib users suffered MIs or sudden cardiac death, compared with just one in the naproxen group. Internal Merck documents (see Table 1) show that the adjudication of events was not without controversy. Some serious adverse events (arterial rupture and death, and hypertensive heart disease and death) suffered with rofecoxib were not ultimately adjudicated as MIs. Most clinicians would have a high level of suspicion of MI for all vascular events leading to sudden death. In any case, the combined endpoint of MI or death for rofecoxib and naproxen in ADVANTAGE was actually as large as 9 to 1 (p<0.03). Given the previous concerns over VIGOR, and given the uncertainties involved in the adjudication procedures, additional attention and transparency would have been appropriate in place of the article’s curt dismissal of MI risk with the comment “p>0.2”. The accurate reporting of this data may have made the safety picture of rofecoxib less misleading until the drug’s “voluntary” removal from the market in September 2004.
| Table 1a Cardiovascular Serious Adverse Experiences Referred for Adjudication in the ADVANTAGE study | ||
| AN | SAE term | Adjudicated Diagnosis |
| Rofecoxib | ||
| 4746 | Ventricular Tachycardia | MI |
| 3700 | MI | MI |
| 0047 | MI | MI |
| 2176 | Acute MI, Coronary Artery Disease | MI |
| 4049 | MI | Sudden/ Unknown Death |
| 5108 | Congestive Heart Failre, Non-Q-MI, Coronary Artery Disease | MI |
| 5382 | Age Indeterminate MI, Unstable Angina, Atrial Fibrillation | MI |
| 1955 | Cerebral Aneurysm, Subarachnoid Hemorrhage, Pulmonay Edema | Hemorrhagic CVA Unstable
Angina |
| 3423 | Arterial Rupture and Death | — |
| 5005 | Hypertensive Heart Disease and Death | — |
| Naproxen | ||
| 0665 | Age Indeterminate MI, Ventricular Fibrillation, Cardiac Arrest | MI |
| 2182 | Transient Ischemic Attack | Ischemic CVA |
| 3155 | Cerebellar Hemorrhage, Cerebrovascular Accident, Chorea | Ischemic CVA |
| 5761 | Cerebral Infarction | Ischemic CVA |
| 6480 | Cerebrvascular Accident | Ischemic CVA |
| 3189 | Cerebrvascular Accident | Ischemic CVA |
| 6099 | Neurologic Disorder | Ischemic CVA |
| aThis table is an extract from a January 19, 2001 letter from Dr Silverman, Merck Senior Director of Regulatory Affairs, to the FDA. | ||
In any discussion of ADVANTAGE, it is important to remember its arguably most disingenuous element, namely that the study had no true scientific hypothesis. Rather, it was designed by the Merck marketing department as a seeding trial to encourage physicians to familiarise themselves with rofecoxib and its prescription. Even the Merck director of research had concluded in internal e-mails that the ADVANTAGE trial had no scientific merit (8). Notably, four of the ADVANTAGE authors were Merck employees and the first author, an academic professor, reported receiving consultation fees from Merck. In fact, the New York Times (8) later quoted this author as saying: “Merck designed the trial, paid for the trial, ran the trial. Merck came to me after the study was completed and said, ‘We want your help to work on the paper.’ The initial paper was written at Merck, and then it was sent to me for editing.”
These facts strongly support the notion that the Vioxx scandal was not a “one-off”, involving one manuscript and one journal. Rather, it appears to have been a concerted effort involving the sponsor, journal editors, peer reviewers and conflicted academic consultants to manipulate an inefficient and amoral scientific process, with the aim of camouflaging, or at least minimising, the cardiovascular risk of rofecoxib for a gullible medical readership. The VIOXX scandal was an abject failure of governance in medical publishing that ultimately put public safety at risk. The transgressions included rapid publication to suit the industry’s needs, superficial peer-reviewing that allowed invalidated hypotheses to be circulated as veracities, obfuscation of clinical data, and pervasive conflicts of interest, leading to misleading inferences and conclusions. Conflicted academic physicians provided a veneer of respectability to these questionable activities, further misleading the medical readership. However, ultimately the responsibility must lie with us, the medical readership, to maintain and enhance our critical assessment abilities and to insist on complete transparency in data accessibility.
Have we learned any lessons from the Vioxx scandal? If so, are the lessons still pertinent today or is this scandal only of historical interest? I would argue that the Vioxx cautionary lessons are, if anything, more germane today as the interplay between industry, academia and medical publishing has become more intertwined and there is greater potential for conflicts of interest. Both universities and funding agencies are now relentless in their push for academics to “partner” with industry. The current research paradigm involves a bench-to-bedside-to-business model and without appropriate safeguards, future scandals such as the Vioxx scandal are perhaps only as distant as the next investigative report. There have been positive steps, including the enactment of the Physician Sunshine Act, to improve transparency regarding financial conflicts of interest between physicians and industry. However, as has been convincingly argued by some (9), while this is a necessary step, it is not sufficient. Professional advancement and recognition for personal achievement, which are non-financial conflicts of interest, may also present problems (10), even if less pernicious than financial conflicts. Given the extent and complexities of the current association of academics and industry, it is surprising that the NEJM editorial board should attempt to push the agenda away from a serious confrontation of financial conflicts of interest with a three-part series (11, 12, 13) which largely repudiates the NEJM’s previous editors’ attempts to manage this issue. On the other hand, given that the NEJM reportedly received approximately $700,000 from Merck for VIGOR reprints, perhaps the change in attitude is not so surprising.
Recently, there has also been a movement to allow open data access as another means of addressing the issues raised by Vioxx and similar scandals. While there has been a general acceptance of data sharing, some journals have displayed a distinct lack of enthusiasm, with the NEJM even employing the disparaging term “research parasites” (14) to refer to those who re-analyse open data. Even those who claim to support data sharing (15) have expressed concerns that primary researchers will not continue their work if their data are effortlessly shared, that the nuances of the data will be missed, and that false conclusions might be formulated if the primary investigators do not oversee who has access to the data and do not play an active role in the control of data sharing (15). An examination of the three studies discussed above (2, 3, 6) reveals how specious this argument is with regard to industry-sponsored data collection. Most commercial studies are largely designed by industry; data collection and analysis are often undertaken by industry independently of the so-called primary academic investigators. Requiring data sharing to be vetted by these primary researchers, who are likely to be closely aligned with the sponsors, is unlikely to produce genuine data sharing and may constitute a future impediment to independent validation of the primary analyses.
Hopefully, the Vioxx scandal has made the medical commons more sceptical and critical of industry-sponsored trials. Hopefully, the Vioxx scandal will also help inform the debate about financial conflicts of interest and the need for complete data sharing. Hopefully, learning these lessons will prevent a repetition of history. Hopefully.
Funding and conflict of interest
Dr Brophy receives salary support from the Fonds de Recherche du Quebec – Sante (FRQS), a non-profit provincial funding agency. In 2007, he served as a consultant to US attorneys representing Vioxx plaintiffs. He has no other relationships that could be construed as a conflict of interest since that time.
Note
1* Merck internal email, August 17, 2001 from Dr Morrison (MRK-ACF0005698)
References
- Wilson M. The New England Journal of Medicine: commercial conflict of interest and revisiting the Vioxx scandal [Internet]. Indian J Med Ethics. Published online on June 15, 2016 [cited 2016 Aug 4]. Available from: http://ijme.in/index.php/ijme/article/view/2407/4974
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343(21):1520-8, 2 p following 1528. doi: 10.1056/NEJM200011233432103. PubMed PMID: 11087881.
- Konstam MA, Weir MR, Reicin A, Shapiro D, Sperling RS, Barr E, Gertz BJ. Cardiovascular thrombotic events in controlled, clinical trials of rofecoxib. Circulation. 2001;104(19):2280-8.
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286(8):954-9.
- Brochier ML. Evaluation of flurbiprofen for prevention of reinfarction and reocclusion after successful thrombolysis or angioplasty in acute myocardial infarction. The Flurbiprofen French Trial. Eur Heart J. 1993;14(7):951-7. PubMed PMID: 8375421.
- Lisse JR, Perlman M, Johansson G, Shoemaker JR, Schechtman J, Skalky CS, Dixon ME, Polis AB, Mollen AJ, Geba GP; ADVANTAGE Study Group. Gastrointestinal tolerability and effectiveness of rofecoxib versus naproxen in the treatment of osteoarthritis: a randomized, controlled trial. Ann Intern Med. 2003;139(7):539-46. PubMed PMID: 14530224.
- Braunstein N, Polis A. Report of specific cardiovascular outcomes of the ADVANTAGE trial. Ann Intern Med. 2005;143(2):158-9.
- Berenson A. Evidence in vioxx suits shows intervention by Merck officials [Internet], April 24, 2005 [cited 2016 Aug 4]. Available from: http://www.nytimes.com/2005/04/24/business/evidence-in-vioxx-suits-shows-intervention-by-merck-officials.html?_r=0.
- Wilson M. The Sunshine Act: commercial conflicts of interest and the limits of transparency. Open Med. 2014;8(1):e10-3.eCollection 2014. PubMed PMID: 25009680; PubMed Central PMCID: PMCPMC4085090.
- Lo B, Field MJ. Committee on Conflict of Interest in Medical Research, Education, and Practice. Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice. Washington (DC): National Academies Press (US); 2009.
- Rosenbaum L. Beyond moral outrage–weighing the trade-offs of COI regulation. N Engl J Med. 2015;372(21):2064-8. doi: 10.1056/NEJMms1502498. PubMed PMID: 25992752.
- Rosenbaum L. Understanding bias–the case for careful study. N Engl J Med. 2015;372(20):1959-63. doi: 10.1056/NEJMms1502497.PubMed PMID: 25970055.
- Rosenbaum L. Conflicts of interest: part 1: Reconnecting the dots–reinterpreting industry-physician relations. N Engl J Med. 2015;372(19):1860-4. doi: 10.1056/NEJMms1502493. PubMed PMID: 25946288.
- Longo DL, Drazen JM. Data sharing. N Engl J Med. 2016;374(3):276-7. doi: 10.1056/NEJMe1516564. PubMed PMID: 26789876.
- Haug CJ. From patient to patient – sharing the data from clinical trials. N Engl J Med. 2016;374(25):2409-11. doi: 10.1056/NEJMp1605378. PubMed PMID: 27168009.
