COMMENT
Truth in research labelling
John H Noble Jr
Published online: December 28, 2016
DOI: https://doi.org/10.20529/IJME.2017.035
Abstract
This report describes the background and context of a currently circulating petition to the US Congress that seeks amendment of Section 801 of the Public Health Services Act (42 U.S.C. 282) to close a loophole in existing law which makes possible post hoc adjustment of randomised controlled trial (RCT) results reported to the Food and Drug Administration that differ from those reported to ClinicalTrials.gov and to medical journals. The report describes the petition’s rationale, underlying assumptions, and support for its proposed remedy in deontological, consequentialist, and casuist philosophical ethics theories. It addresses the several reservations of the World Association of Medical Editors (WAME) with citations of evidence for the petition’s assertions. The report suggests that some medical journals are not unknowing victims but rather complicit enablers of the post hoc adjusted RCT results that they publish. Its closing remarks dwell on the negative impact that embrace of a neoliberal, anti-regulatory philosophy of government will likely have on any regulatory reform to promote the integrity of biomedical science and the future of evidence-based medicine.
Background and context
Almost daily the media report discovery of fraudulent or misleading biomedical research in which the responsible parties are typically identified as either industry employees or their agents. One such report documented more than 65,000 product liability lawsuits – more than in any other industry – and suggested that the FDA and the US Department of Justice were inattentive to patient safety, despite settlements in criminal and civil liability cases with virtually every major drug manufacturer (1). Noble asked why this was happening since statisticians and researchers have been educated to understand the rules of scientific enquiry. He concludes,”. . . in the vast majority of cases not to play by the rules is the conscious choice of a knave, not a fool” (2: p 28).
One can reasonably ask, therefore, if corruption is seeping into the culture of biomedical research and contaminating the published content of its journals. And if so, what are the consequences for physicians and their patients in terms of avoidable injuries, illnesses, and deaths? Peter Gotzsche concludes with an abundance of detailed evidence that the pharmaceutical and medical device industry has corrupted healthcare and boldly compares its business model with organised crime (3).
Recently there has been a spate of reports connecting industry advertising and medical journals. Yet Richard Smith, former editor of the British Medical Journal, made that connection as early as 2005 and 2006 in two provocative insider revelations – one entitled “Medical journals are an extension of the marketing arm of pharmaceutical companies” and the other, “Patient safety requires a new way to publish clinical trials” (4, 5).
Clearly, Smith’s early call for medical journals to cease publishing clinical trial results was ignored. Nothing came of his recommendations for posting trial protocols and interim reports on the Web to enable “open debate about the importance, relevance, and quality of the trial,” nor when the trial was finished, for uploading the full data set with provision for preprogrammed statistical analysis of results to preclude post hoc manipulation by authors of clinical trial results. Had Smith’s recommendations been adopted, the capacity of medical journals would have been enhanced for protecting themselves from inaccurate and misleading claims about clinical trial procedures and results.
The folly of ignoring Smith’s advice is revealed by persistent reports of grossly inaccurate reporting of randomised controlled trial (RCT) results and their adverse effects on the health and well-being of patients and the continuing resistance of medical journal editors to retracting published reports or even acknowledging that a problem exists.
Mark Wilson’s recent revisit to the Vioxx scandal and the role that commercial conflict of interest played has put the New England Journal of Medicine (NEJM) at the epicentre of new criticisms relating to other instances of the journal’s failures (6). He provides a heavily documented account of NEJM editor Jeffrey Drazen’s interactions with two physicians who wrote a letter to make known the existence of a greater number of Vioxx-related adverse events than indicated by the NEJM report of the Vigor study group (7). Drazen’s dismissive retort to the physicians, “We can’t be in the business of policing every bit of data we put out,” was gratingly insensitive (8).
James Brophy followed up with additional evidence of medical journal failure to vet properly manuscripts submitted for publication (9). In order to counter a forthcoming cautionary JAMA article, the specialty journal Circulation had published a meta-analysis of cardiovascular thrombotic events in controlled, clinical trials of rofecoxib within the absurdly brief timeline of 24 hours between submission and acceptance and electronic publication 12 days later (10). He challenged the journal’s peer-review process or that of any other scientific journal to provide 24-hour turnaround, intimating the expediency of the journal’s editors in response to the sponsor’s “urgent need for quick publication”
Ruth Macklin, in turn, recounts her own personal struggles to publish a one-page letter in opposition to the NEJM publication of Benjamin Wilfond and 45 others defending the use of consent forms in the controversial SUPPORT trial that was rebuked by the Office of Human Research Protection (11, 12). The back-and-forth between Macklin and the NEJM editors provides a case study in passive aggressive stalling, followed by acceptance of her letter subject to the addition of four citations of NEJM reports favouring the position that Macklin opposes.
Arguably, the NEJM had crossed the line between neutral impartiality and active advocacy by advancing the utility preferences of a client. One can reasonably ask if sophisticated readers will now read the NEJM and take the political economic perspective: “Who benefits and who pays?” Does this publication reflect the expected conservative ideology and advocacy of an American Enterprise Institute or a Stanford University Hoover Institution or the expected liberal ideology and advocacy of a Brookings Institution?
The sign of a political agenda is journal publication of an editorial listing the names of interest group members. Oransky and Marcus describe the behaviour of the NEJM editors in this regard (13). The NEJM took aim at the International Committee of Medical Journal Editors, of which Drazen is a member, for proposing that data from RCTs be made available within six months of publication. It gathered 280 researchers from more than 30 countries to sign an editorial asserting that open access to data for verification and replication purposes would slow scientific progress and endanger public health.
The NEJM position, in effect, denied the validity of intersubjective verifiability – the core principle of empirical science (14, 15). It rejected the commonsense proposition that published claims cannot be accepted without looking at the data and the methods employed to obtain and analyse them.
There is ongoing dispute between some medical journals and the watchdog group COMPare about acceptable practice in the reporting of RCT outcomes. At issue is transparency in making explicit any changes between the original protocol and what gets reported and published by a medical journal. The convoluted exchange between COMPare and the medical journals began, as Peter Doshi recounts, with a confrontation:
Over six weeks, Goldacre’s [COMPare] team analysed 67 trials published across five leading medical journals–Annals of Internal Medicine, The BMJ, JAMA, Lancet, and the New England Journal of Medicine (NEJM). Only nine trials, according to COMPare, were “perfect,” meaning the publication reported all prespecified outcomes and flagged for readers any changes from the protocol (such as introducing outcomes after the trial started). Between them the 58 offending trials failed to report 354 specified outcomes and “silently added” an almost equal number (357). The team dispatched 58 letters to the journal editors seeking correction to the published record. However, few corrections resulted (16)
There are recent reports that name the ghostwriters, the paying pharmaceutical companies, and the paid big-name academics who misrepresent themselves as authors of the industry-procured, ghost-written medical journal reports. Cosgrove, Vannoy, Mintzes, and Shaughnessy vividly describe the interplay among industry, publishing, and FDA drug regulation (17). There is enduring truth in the saying, “Whose bread I eat, his praise I sing.” The critical question is, “Are medical journals being unknowingly deceived or are they complicit?” Either way, it is dismal commentary on some medical journals and their peer-review process . . . and perhaps an explanation for journal resistance to full transparency in the reporting of results of RCTs.
Amsterdam, McHenry and Jureidini provide damning evidence that the medical journals are complicit rather than deceived, stating “medical journals and their owners have become dependent upon pharmaceutical revenue, whereby they fail to adhere to the standards of science” and “rarely publish critical deconstructions of ghostwritten clinical trials” (18).They provide three case studies of litigation involving ghostwriting – two relating to GlaxoSmithKline’s Paroxetine Study 329 and Study 352 and one relating to Forest Laboratory’s Citalopram Study CIT-MD-18. At issue in all three cases was selective reporting of outcomes with suppression of those indicating negative results.
To their credit, some publishers are reacting to “ghosting” and other manipulations of journals. Springer, MC, for example, has retracted 58 papers on “evidence of plagiarism, peer review and authorship manipulation” and “attempts to subvert the peer-review and publication system to inappropriately obtain or allocate authorship” (19).This action by Springer, MC, however, is unusual. Alistair Matheson paints a different picture of righteous posturing by the publishing industry accompanied by sly redefinition of “ghostwriting” to make it go away if footnote mention is made to the ghostwriter for “editorial assistance” or a similar euphemism (20).
Matheson’s follow-up BMJ Rapid Response account of his attempts to obtain clarification from the three leading trade associations of the commercial publications industry and its prime trade advocacy organisation – the International Society for Medical Publication Professionals (ISMPP), the European and American Medical Writers Associations (EMWA and AMWA), and the Global Alliance of Public Professionals (GAPP) – reveal a self-protective circling of wagons. He describes the results of his attempts to clarify the trade associations’ joint statement on transparency in the commercial publications trade as failing to elicit:
. . . any details of the commercial publications plans, scientific platforms, product positioning and key messages underpinning its past, and future, journal articles; nor did it agree to release details about its recruitment and use of “key opinion leaders”; nor details of intellectual property rights; nor any indication of how much money its companies receive from industry clients to plan and develop these articles (20).
The launch of a new petition
Against this background and context of entrenched interests, Bernard Carroll, John Noble, and John Nardo have collaborated with the Science and Evidence Council of the Lown Institute’s Right Care Alliance to circulate a petition to the US Congress seeking remedy for persistent misrepresentations of the results of RCTs (21). It would require full transparency in reporting the nature of the RCT data and analyses on which claims of safety and efficacy of new drugs and medical devices are based.
Underlying the petition is the assumption that researchers fully understand the rules of scientific enquiry. They know how to design and conduct reliable and valid RCTs. They understand the scientific requirement of making known at the outset before beginning recruitment of human research subjects the necessity of specifying the causal theory and its derivative hypotheses, the operational definitions or measures of the factors that will produce the outcomes predicted by the theory, and a plan for statistically analysing the collected measures of the outcomes. They understand that deviations from this original statement or protocol should be labelled as such to alert other researchers and general readers about any adjustments that are made to the original statement or protocol and why. In this way, it would become possible for others to verify and replicate the reported outcomes.
The petition goes to some length in making known how industry and some researchers go about breaching the rules of scientific enquiry to produce biased and misleading claims of efficacy and safety. It formulates corrective action via amendment of the existing legislation that requires registration of all RCTs. The amendments require: (a) responsible parties to provide identical RCT informational content to the FDA in support of an application for marketing approval and to the ClinicalTrials.gov registry that serves as a conduit of information to a variety of stakeholders, including physicians and patients, and (b) to report RCT results as either (i) strictly conforming to the registered FDA submission or (ii) containing post hoc adjustments to it.
Such “truth in research labelling” is meant to curb current abuses achieved through reporting different RCT results to the FDA than reported to ClinicalTrials.gov and to medical journals. Its modest goal is to correct a major flaw in existing legislation that permits gaming the system to thwart the intended beneficial effects of registering all clinical trials and faithfully reporting their true results in a timely manner. The petition does not address the wider problem of scientists behaving badly and the threat it poses to the good reputation and public support of science (22, 23, 24).
Ethics support for the petition
The petition seeks support by members of the US Congress and other stakeholders – particularly, physicians and their patients – by appealing to the Kantian moral imperative of “truth telling”. Deontological ethics theories assert principles or rules as guides to the behaviour of moral agents (25).The most common examples in bioethics are the Hippocratic Oath, the Nuremberg Code, the Declaration of Helsinki, and the Belmont Report. Kant argued that “truth telling” in keeping promises is the bedrock requirement for enabling sustainable interpersonal transactions among members of society (26).
The petition also makes consequentialist arguments based on examples of what happens when truth telling is compromised for reasons of personal or corporate gain to the detriment of others for whom a duty is owed to enhance their welfare or at least not to cause harm. The petition points out the beneficial consequences that might be brought about by adoption of the proposed “truth in research labelling” amendments to Section 801 of the Public Health Service Act. In this it is consistent with the frequent resort to utilitarian weighing of benefits and harms that characterises contemporary bioethics.
The petition’s reliance on deontological and consequentialist ethics theories combined with case examples to justify taking corrective action draws on the case-based reasoning of casuist ethics. The petition’s ethics-based arguments thus reflect the notion of philosophy in bioethics as “bricolage,” that is, a synthesis from diverse sources which takes stock of “available conceptual resources, and then attempts to solve the problem by taking things apart, reordering, culling out, weighing, specifying, splicing in, and putting them all back together” (27: p75).
World Association of Medical Editors’ (WAME) reservations and responses
A copy of the petition to the US Congress was shared with WAME. While the WAME Executive Committee expressed agreement with the underlying proposal that the FDA and NIH should assure consistency in reported data, it withheld endorsement because it believed the petition contained unsubstantiated statements (28). Further, WAME, as an international organisation, determined it would not distribute the petition to its members because the problem and issue is “primarily” US-based.
Response: It is good that we agree on the need to assure consistency in reported data. It is understandable that WAME, as an international organisation, would want to avoid becoming involved in the internal affairs of the United States. Our petition could nevertheless serve as a model for other jurisdictions, should they wish to take remedial action.
WAME specified six instances of unsubstantiated assertions in the petition:
- The petition states, “This weak oversight by two Federal agencies, and their lack of coordination, has led over time to a high frequency of misleading scientific reports in medical journals (citations 6–8).”
- WAME states, “The problem exists but the frequency is not known.”
- The petition states, ” . . . editors and reviewers are currently unable to perform effective peer-review of corporate clinical trials reports” with the WAME asserted implication that “any existing published pharma-funded studies are unreliable based on ineffective peer-review, which is not a known fact and would undermine substantial portions of the medical research literature.”
- The petition states, “Most corporate publications in medical journals address secondary questions that the original clinical trials were not designed to answer, using biased in-house statistical analyses that neither the FDA nor any other external agency ever reviewed or approved” with the WAME rejoinder that “‘Most corporate publications’ require the evidence behind this statement. Certainly some, or even many, may, but the idea that most corporate publications were of analyses not specified in the protocol needs more substantial evidence than an assertion.”
- The petition states, “We urge an explicit provision that Results be posted on ClinicalTrials.gov at the time of any submission for publication” with the WAME rejoinder that “This requirement creates the potential for enforcing the creation of inconsistencies between clinicaltrials.gov and the eventual published article, since peer and editorial review can identify inconsistencies and lack of clarity in results reporting that may lead to changes in results prior to publication.”
- The petition states, “Rather by providing a means for external verification that submitted manuscripts are faithful to the a priori protocols and plans of analysis, this proposal frees the journals from an investigative duty for which they are not equipped and at which they regularly fail” with the WAME rejoinder that “Again, ‘regularly fail’ is not known from the established instances.”
Response: The petition citations 6–8 were to two highly respected former medical journal editors – BMJ editor Richard Smith and NEJM editor Marcia Angell – and to the current Lancet editor Richard Horton. We relied on the published documentation and insights of these “insiders” in our assertion.
We felt our assertion was also dramatically substantiated by the petition’s graphic display of the findings of Turner and colleagues (29) below:
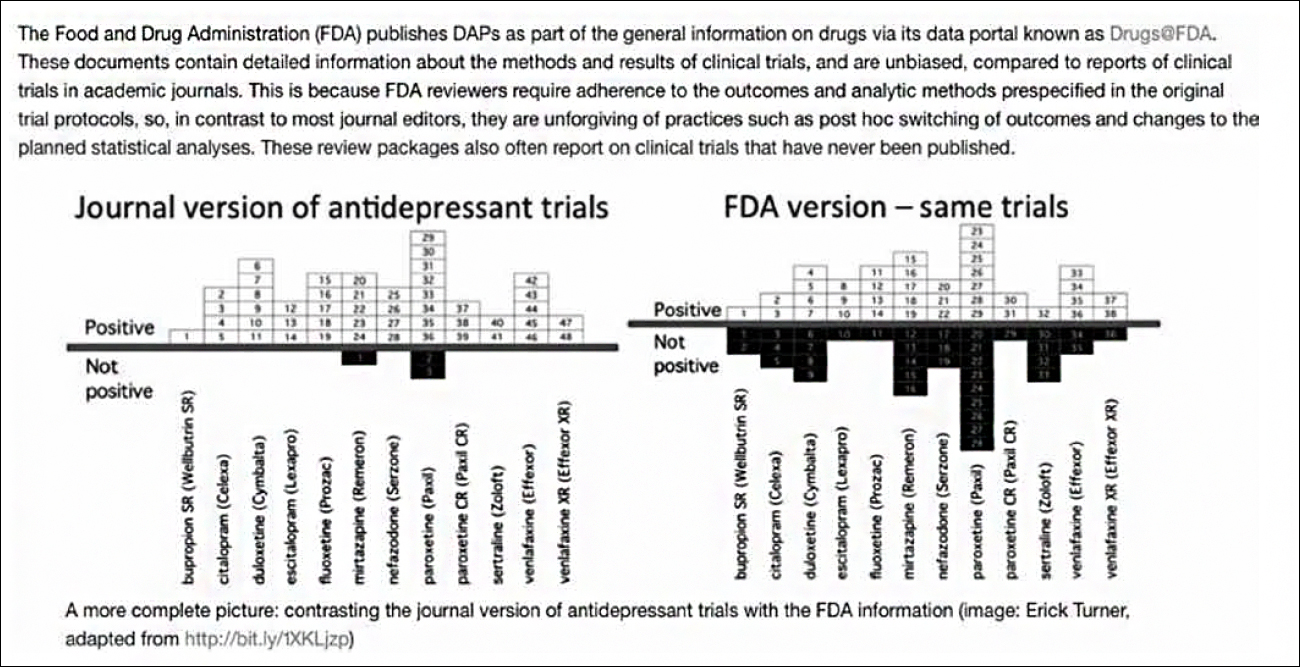
Response: We agree the problem exists and, unfortunately, the precise frequency is unlikely to ever be known in face of numerous obstacles to accurate reporting. We must depend on assembling published and unpublished reports from a variety of sources, such as the study by Turner and colleagues (29) of the discrepancies between medical journal and FDA versions of the same trials. What goes on out-of-sight is only beginning to emerge in the aforementioned recent reports by Wilson (6), Brophy (9), Macklin (11), Doshi (16), Cosgove, Vannoy, Mintzes and Shaughnessy (17), Amsterdam, McHenry and Jureidini (18), McCook (19), and Matheson (20).
Response: We do not assert “any”, implying “none.” Nonetheless, we stick with our assertion because of accumulating evidence about biased and misleading pharma-funded reporting that is sometimes “ghost” written in violation of generally accepted ethical norms for university faculty behaviour. Litigation and whistleblower disclosure of behind-the-scene scheming and manipulation by pharmaceutical companies substantiates our skepticism about the ability of editors and reviewers to perform effective peer-review.
When the manuscript comes listing as authors a string of academic stars and medical key opinion leaders, it is understandable that journal editors would leap at the opportunity to publish some new “break-through” finding. Are we not all human?! Again, we refer back to our list of cited sources for recent reports of behind-the-scene pharmaceutical company scheming and manipulation.
Regrettably, a substantial portion of the medical research literature has already been assessed and found wanting. Sir Iain Chalmers, one of the founders of the Cochrane Collaboration, and John Ioannidis have published evidence that as much as 85% of the investment in biomedical research worldwide is preventable waste and an untrustworthy guide to evidence-based medicine (30, 31). So, how can publication of its results be otherwise?
Response: Agreed, “many” rather than “most” would have been the better characterisation in face of the lack of difficult-to-attain precise statistics. Again, we cite in support of our corrected assertion of “many” the aforementioned publications of Wilson (6), Brophy (9), Macklin (11), Doshi (16), Cosgove, Vannoy, Mintzes and Shaughnessy (17), Amsterdam, McHenry and Jureidini (18), McCook (19), and Matheson (20).
Response: Would that the content posted on ClinicalTrials.gov were up to supporting peer and editorial review that would make possible identification of “inconsistencies and lack of clarity in results reporting that may lead to changes in results prior to publication”! All evidence points to limited compliance with the Section 801 mandate of the Public Health Service Act and no relief in sight without coordination between the FDA and NIH to assure identical RCT content submitted to the FDA in support of marketing approval and to ClinicalTrials.gov – the goal of our petition to the US Congress (32, 33).
That said, the petition does not prevent or discourage peer and editorial review of submitted manuscripts for inconsistencies and lack of clarity. Further, requiring changes in results reporting prior to publication – if justified – is a good thing . . . provided they are flagged as post hoc adjustments in the original RCT content that was submitted to the FDA in support of marketing approval. Indeed, it is also good for FDA reviewers of original RCT content to receive, when needed, a second outside opinion.
Response: If, as noted previously, all evidence points to limited compliance with the Section 801 mandate and, therefore, no basis for supporting peer and editorial review, how is it possible to not “regularly fail”? The information needed to judge “inconsistencies and lack of clarity in results reporting that may lead to changes in results prior to publication” simply does not exist. But more disturbing is evidence that when instructing peer reviewers to evaluate RCT results, medical journal editors ignore expert opinion about what to look for (34).
Closing observation
The results of the US presidential election giving one party control of the executive and legislative branches of government predict that decision-making will reflect a neoliberal, anti-regulatory philosophy of the government. The American healthcare system and the biomedical research enterprise will feel the effects.
Under these circumstances acceptance by the US Congress of our proposed “truth in research labelling” amendments to Section 801 of the Public Health Service Act seems unlikely to happen in the foreseeable future. Meanwhile, other countries may find the approach useful, should they wish to take action to promote the integrity of biomedical science and the future of evidence based medicine.
Acknowledgements
I wish to acknowledge the helpful critiques and suggestions for improvement by Mark Wilson, Bernard Carroll, and John Nardo of early drafts of this comment.
References
- Schmidt J. More drugs slapped with lawsuits. USA Today. August 23, 2006 [cited 2016 Dec 3]. Available from: http://usatoday30.usatoday.com/money/industries/health/drugs/2006-08-23-drug-lawsuits-usat_x.htm.
- Noble Jr JH. Detecting bias in biomedical research: looking at study design and published findings is not enough. Monash Bioeth Rev. 2007;26(1-2):24-45.
- Gotzsche PC. Deadly medicines and organised crime. London: Radcliffe Publishing; 2013.
- Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005 [cited 2016 Dec 3];2(5):138e. Available from: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0020138.
- Smith R, Roberts I. Patient safety requires a new way to publish clinical trials. PloSClin Trials. 2006 [cited 2016 Dec 3];1(1):e6. Available from: http://journals.plos.org/plosclinicaltrials/article?id=10.1371/journal.pctr.0010006.
- Wilson M. The New England Journal of Medicine: commercial conflict of interest and revisiting the Vioxx scandal. Indian J Med Ethics online. June 15, 2016 [cited 2016 Dec 3]. Available from: http://www.ijme.in/index.php/ijme/article/view/2407/4974.
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MP, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343(21):1520-8, 2 p following 1538.
- Armstrong D. Bitter pill: how the New England Journal missed warning signs on Vioxx. Wall Street Journal [internet]. May 15 2006 [cited 2016 Dec 3]. Available from: http://www.wsj.com/articles/SB114765430315252591.
- Brophy JM. Vioxxredux—or how I learned to worry about industrysponsored clinical trials. Indian J med Ethics online. August 30, 2016 [cited 2016 Dec 3]. Available from: http://www.ijme.in/index.php/ijme/rt/printerFriendly/2458/5043.
- Konstam MA, Weir MR, Reicin A, Shapiro D, Sperling RS, Barr E, Gertz BJ. Cardiovascular thrombotic events in controlled, clinical trials of rofecoxib. Circulation. 2001;104(19):2280-8.
- Macklin R. Conflict of interest and bias in publication. Indian J Med Ethics online. August 8, 2016 [cited 2016 Dec 3]. Available from: http://ijme.in/index.php/ijme/article/view/2451/5034.
- Wilfond BS, Magnus D, Antommaria AH, AppelbaumP, Aschner J, Barrington KJ, Beauchamp T, Boss RD, Burke W, Caplan AL, Capron AM, Cho M, Clayton EW, Cole FS, Darlow BA, Diekema D, Faden RR, Feudtner C, Fins JJ, Fost NC, Frader J, Hester DM, Janvier A, Joffe S, Kahn J, Kass NE, Kodish E, Lantos JD, McCullough L, McKinney R Jr, Meadow W, O’Rourke PP, Powderly KE, Pursley DM, Ross LF, Sayeed S, Sharp RR, Sugarman J, Tarnow-Mordi WO, Taylor H, Tomlinson T, Truog RD, UnguruYT, Weise KL, Woodrum D, Youngner S. The OHRP and SUPPORT. N Engl J Med. 2013;368(25):e306.doi: 10.1056/NEJMp1307008.
- Oransky I, Marcus A. NEJM editorial doubles down on resistance to data sharing. Stat News. August 10, 2016 [cited 2016 Dec 3]. Available from: https://www.statnews.com/2016/08/10/.
- Ayer AJ. Language, truth and logic. New York: Dover Publications; 1936.
- Wikipedia. Intersubjective verifiability, January 29, 2016 [cited 2016 Dec 3]. Available from: https://en.wikipedia.org/wiki/Intersubjective_verifiability.
- Doshi P. Is this trial misreported? Truth seeking in the burgeoning age of trial transparency. BMJ. 2016 [cited 2016 Dec 3];355:i5543. Available at: http://www.bmj.com/content/355/bmj.i5543?ijkey=10h2T9z3F0g7HA4&keytype=ref.
- Cosgrove L, Vannoy S, Mintzes B, Shaughnessy AF. Under the influence: the interplay among industry, publishing and drug regulation. Account Res. 2016 [cited 2016 Dec 3];23(5):257-79. Available from: https://www.researchgate.net/publication/295077256_Under_the_Influence_The_Interplay_among_Industry_Publishing_and_Drug_Regulation.
- Amsterdam JD, McHenry LB,Jureidini JN. Commentary: Industrycorrupted psychiatric trials. International Network for the History of Neuropsychopharmacology. July 11, 2016 [cited 2016 Dec 3]. Available from: http://inhn.org/controversies/barry-blackwell-corporatecorruption-in-the-psychopharmaceutical-industry/jay-d-amsterdamleemon-b-mchenry-and-jon-n-jureidinis-commentary-industrycorrupted-psychiatric-trials.html.
- McCook A. Springer, BMC retracting nearly 60 papers for fake reviews and other issues. Retraction Watch Blog. November 1, 2016 [cited 2016 Dec 3]. Available from: http://retractionwatch.com/2016/11/01/springer-bmc-retracting-nearly-60-papers-for-fake-reviews-and-otherissues/#respond.
- Matheson A. Ghostwriting: the importance of definition and its place in contemporary drug marketing. BMJ. 2016 [cited 2016 Dec 3];354:i4578. Available from: http://www.bmj.com/content/354/bmj.i4578.
- Petition to the US Congress: “Stop false reporting of drug benefits & harms by making FDA & NIH work together” [cited 2016 Dec 3]. Available from: https://www.change.org/p/congress-congress-stop-false-reporting-ofdrug-benefits-harms-by-making-fda-nih-work-together?tk=DszjcKyouigz0RlIlrQLq4pU7KSx4YIouffgveocPtM&utm_medium=email&utm_source=signature_receipt&utm_campaign=new_signature.
- Martinson BC, Anderson MS, deVries R. Scientists behaving badly. Nature. 2005;435(7043):737-8.
- Spears T. Ethics journal helps scientists cheat their way to success. Ottowa Citizen. September 28, 2016 [cited 2016 Dec 3]. Available from: http://ottawacitizen.com/technology/science/predatory-journalsreach-new-frontier-ethics.
- Cookson D. Misconduct pervades UK research. Financial Times. January 12, 2012 [cited 2016 Dec 3]. Available from: https://www.ft.com/content/bc6f7204-3d1f-11e1-8129-00144feabdc0.
- Frankena W. Ethics, 2nd ed. Englewood Cliffs. NJ: Prentice Hall; 1973.
- Kant I, Gregor MJ (translator). Groundwork of the metaphysics of morals. New York: Cambridge University Press; 1997.
- Stout J. Ethics after Babel: the languages of morals and their discontents. Boston: Beacon Press; 1988.
- Personal communication with WAME secretary Margaret Winker Cook, September 30, 2016.
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthall R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008 [cited 2016 Dec 3];358:252-60. DOI: 10.1056/ NEJMsa065779. Available from: http://www.nejm.org/doi/full/10.1056/NEJMsa065779.
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009 [cited 2016 Dec 3];374(9683):86-89. Available from: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(09)60329-9/abstract.
- Ioannidis JPA. How to make more published research true. PLoS Med. 2014 [cited 2016 Dec 3];11(10):e1001747. doi:10.1371/journal.pmed.1001747. Available from: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001747.
- Riveros C, Dechartres A, Perrodeau E, Haneef R, Boutron I, Ravaud P. Timing and completeness of trial results posted at ClinicalTrials.gov and published in journals. PLoS Med. 2013 [cited 2016 Dec 3];10(12):e1001566. Available from: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001566.
- Silverman, E. What clinical trial results? Now you can see who isn’t sharing their findings. Stat News. November 1, 2016. [cited 2016 Dec 3]. Available from: https://www.statnews.com/pharmalot/2016/11/03/clinical-trials-sanofi-shire-glaxo/.
- Chauvin A, Ravaud P, Baron G, Barnes C, Boutron I. The most important tasks for peer reviewers evaluating a randomized controlled trial are not congruent with the tasks most often requested by journal editors. BMC Medicine 2015; 13: 158. DOI: 10.1186/s12916-015-0395-3 [cited 2016 Dec 5]. Available from: http://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-015-0395-3.
