ARTICLES
National Vaccine Policy: ethical equity issues
Jayakrishnan T
DOI: https://doi.org/10.20529/IJME.2013.054
Abstract
The ministry of health and family welfare published the nationalvaccination policy in April 2011. The policy document drew severecriticism from several public health experts. A review of the print andweb-based literature on the national vaccine policy was done andthe issues of ethics and equity involved in introducing new vaccinesunder the Universal Immunisation Programme (UIP) were studied.
The average coverage of the UIP vaccines at the national levelis below 50%. Despite this, the policy document did not stateany concrete strategy for increasing the coverage. The mainstumbling block for evidence-based vaccine policy in India is thelack of reliable epidemiological data, which makes it difficult forthe National Technical Advisory Group on Immunisation to offersound technical advice to the government. No attempts have beenmade to prioritise diseases or the selection of vaccines. The policysuggests the introduction of the following vaccines in the UIP:Haemophilus influenzae type b, pneumococcal vaccine, rotavirusvaccines and human papillomavirus (HPV). This selection ison the grounds of the vaccines’ availability, not on the basis ofepidemiological evidence or proven cost-effectiveness. This is acritical review of the current vaccination policy and the move toinclude the rotavirus and HPV vaccines in the UIP.
Introduction
Vaccines are important preventive medicines for primaryhealthcare, are critical for a nation’s health security and play auseful role in public health by reducing morbidity and mortalitydue to communicable diseases (1, 2, 3). Every country should haveits own immunisation policy that states how the governmentproposes to universalise the benefits of immunisation to thelarge sections of the population which do not receive thebasic vaccinations, and also describes how new vaccines areto be selected for introduction in the Universal ImmunisationProgramme (UIP) (3, 4).
The ministry of health and family welfare (MOHFW) publishedthe national vaccination policy in April 2011 (5). This policywas drafted by the National Technical Advisory Group onImmunisation (NTAGI), a government – constituted committeeof experts. As for the context and framework of the policy, itstates, “The document covers all categories of vaccines usedin the UIP, vaccines available but not part of the UIP and thosevaccines which are likely to become available in future.” (5: p 4) The chapter on ethics and equity stresses, “The ethicaluse and equitable access to prevention and care should bethe basic mantra of any programme meant for ameliorating disease burden in the country.”(5: p 28) The document alsosuggests the introduction of a few new vaccines in the UIP.When the document was published, it evoked severe criticismfrom several public health experts.
Methods
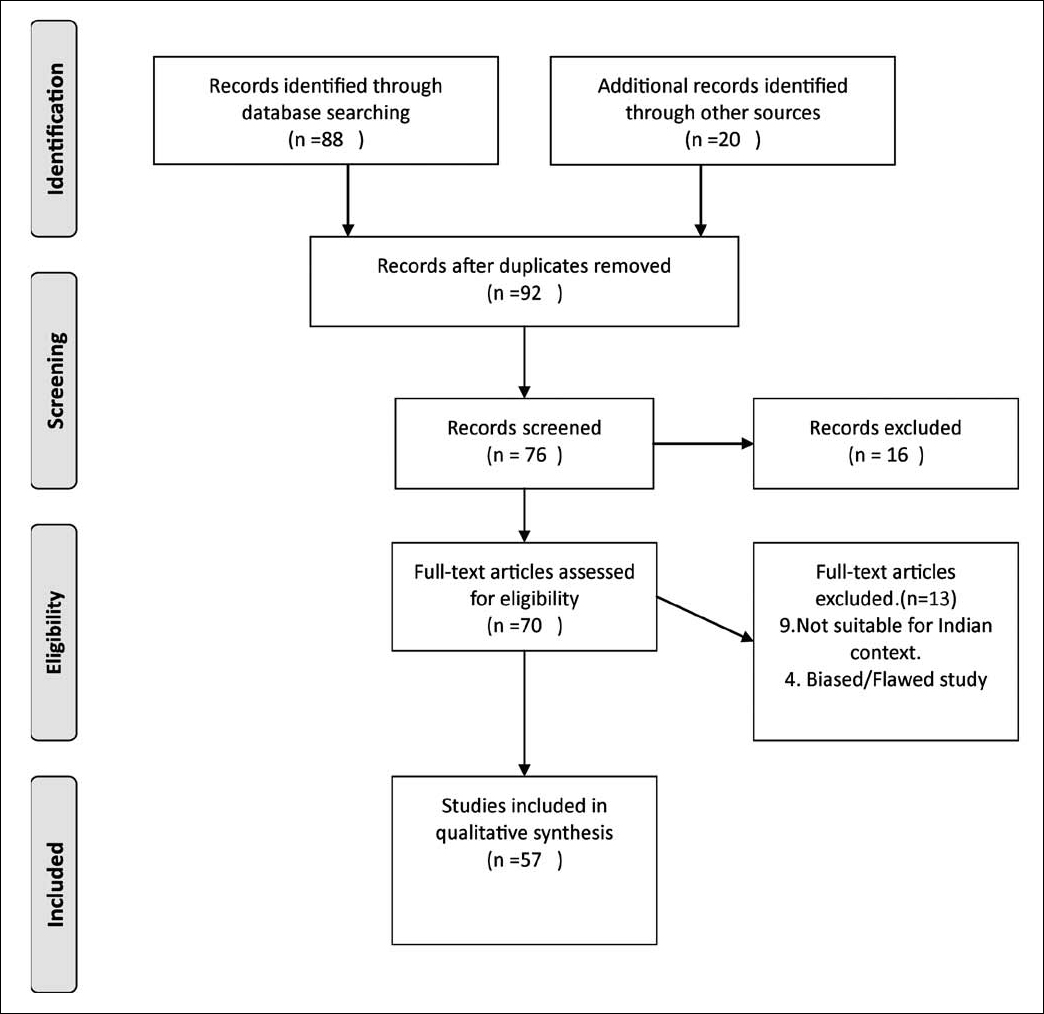
A review of the literature was made through a search of thepublished articles, printed and web-based (Pubmed andGoogle), on the subject from August 1, 2012 to December30, 2012. The keywords or phrases used for the search were’vaccine policy’, ‘cost-effectiveness’, ‘ethical issues in vaccination’,’rotavirus vaccine’, and ‘human papillomavirus (HPV) vaccine’.Various appropriate combinations of the keywords or phraseswere also used. The references of selected articles werescrutinised further to access more literature. Only articlespublished in English from January 2000 to December, 2012,were included in the review (Fig 1). These included bothoriginal studies and review articles (print 14+web 43).
Review of vaccine policy and current UIP
A vaccine policy would indeed be a welcome development ifit succeeded in giving an epidemiologically sound rationale tothe vaccination programme in the country (6, 7). Unfortunatelythe policy draft is non-committal in this respect. It wasformulated following judicial prompting by the Delhi HighCourt during the hearing of a public interest petition thatalleged that the newly introduced vaccines (pentavalent) in thecountry lacked sufficient evidence and asked the governmentto state its policy on vaccines (4).
The UIP in India is one of the largest in the world, targeting27 million infants and 30 million pregnant women everyyear. While Indian manufacturers provide 43% of the globalvaccine supply, the average coverage of the UIP vaccines atthe national level is below 50% (5). Despite this, the policydocument does not put forward a concrete strategy toincrease coverage and does not propose any mechanismto improve the availability of vaccines for the remaining50%, except offering incentives for auxiliary nurse midwives.All doses of the currently included diphtheria, tetanusand pertussis vaccine (DPT) cost less than Rs 15, but theproduction remains erratic and demand-supply gapscontinue. The difference between requirement and supply in 2010-11 was 13.7 million doses for the BCG vaccine and 40.9million doses for the DPT vaccine (50%) (8).The rapid growth(8%-10% per annum) of India’s current vaccine market canbe attributed mainly to the new, high-priced vaccines, anabundance of which are combination vaccines and are notpart of any national programme (2, 6, 7). As for the vaccinesecurity of the country under the current UIP vaccines, thedocument states, “Since there is limited production capacityof vaccines in public sector units (PSU), involvement ofprivate sector manufacture is required to ensure supplyof UIP vaccines.” (5: p 4). There is no word of revamping thePSUs, which have been closed since 2008, or strengtheningthe public sector (9). There is a mismatch between the stated national health policy of self-reliance and self-sufficiency invaccine production; supply remains unaddressed.
Newer vaccines and criteria for inclusion
Vaccines cannot prevent all deaths due to communicablediseases, but rationally selected vaccines can cost-effectivelyreduce the morbidity and mortality associated with someimportant diseases which are epidemiologically relevant for thecountry (2). A well thought-out immunisation schedule mustbe epidemiologically relevant to the country’s health statusand only target serious diseases or public health problemsfor which effective vaccines are available, with others beingdeemed non-universal (10).
The vaccine policy states, “Diseases which are prevalent indeveloping countries are often different than the ones indeveloped countries; majority of the vaccine research isbeing done in developed countries and focus on the diseasesprevalent in those countries.”(5: p 5) The target diseases forvaccines and research need to be modified according to oursituation. The policy should have elaborated on any seriouswork being undertaken by the NTAGI on this subject andshould have prioritised the diseases for vaccine selection inIndia, instead of merely copying from the developed countries.
The criteria for selection of vaccines for the introduction ofnew vaccines in the UIP touch upon disease epidemiologyonly to the extent of mentioning “Disease burden (incidence/ prevalence, absolute number of morbidity / mortality,epidemic / pandemic potential)” (5: p17) and “consideration forpathogen, host and environmental interactions and long-termimpact of vaccination on disease epidemiology have simplygone missing”(6). Epidemiological discussions on guidingpolicy should also consider the burden of a particular diseasecompared to other health problems, as well as the extent towhich the disease can be controlled by vaccination and thepossibility of the development of more serious infection due tostrain replacement, as happened with Haemophilus influenzaetype b (HiB) infection in the West, or age-shifting due to subimmunisation,as in the case of rubella (6, 11).
One of the main stumbling blocks for an evidence-basedvaccine policy in India is the lack of reliable epidemiologicaldata. There are insufficient data on the actual prevalence andincidence of disease, pathogen strain/serotype variations, andimmunity with and without vaccination among populationsof different geographical regions/age groups (2). The policylays down certain guiding principles for the identificationof vaccines/diseases of local relevance as follows: “based oninformation derived from strong surveillance system withincountry. Furthermore, the data from the investigator-initiatedresearches, from modelling studies, and the data from countrieswith either geographical proximity or similar demography mayalso be used.” (5: p16) The current level of disease surveillance inIndia is insufficient to support unequivocal scientific decisionsbased on established principles of public health. Theselimitations severely affect the task of the NTAGI, on whichthe Union Government currently relies for all its vaccination policy decisions (2). The NTAGI depends on extrapolated datafrom studies with small sample sizes based on a few hospitals,blood banks in India, or studies carried out abroad for decisionmaking.The latter confounds the problem and benefits interestgroups attempting to push all available vaccines into thenational programmes, regardless of the necessity, suitability,cost-effectiveness, safety and sustainability of these vaccinesand their bearing on our health priorities (2, 4). Beyond thecriteria named for the selection of diseases of public healthimportance, no attempts have been made to prioritise diseases.Our vaccination policy has suffered a great deal on account ofthese limitations.
As for vaccines to be included in the UIP, the policy declaresthat the following factors need to be considered: “safety andefficacy of the vaccine; affordability and financial sustainabilityof the vaccination programme, even if the initial introduction issupported by an external funding agency; programme capacityto introduce a new antigen, including cold chain capacity;availability of a domestic or external vaccine productioncapacity; the cost-effectiveness of the vaccination programmeand also of the alternatives other than vaccination” (5: p17).Vaccine efficacy and cost-benefit/risk-benefit analysis arerelevant only when the need for a vaccine is proven. However,there is no evidence that these points were considered whileframing the policy.
The policy stresses the introduction of the following vaccines:Hib vaccines, pneumococcal conjugate vaccines, rotavirusvaccines and vaccines for HPV, the burden of which is estimatedto be high, with the vaccine having considerable potential toreduce child mortality. No epidemiological evidence favouringthe introduction of any of these vaccines is available from India (4, 12). An attempt has been made to incorporate the principlesof ethics and equity in the inclusion of newer vaccines bysaying, “The new vaccines which are relatively more expensivethan traditional vaccines are commonly used by the upperand middle class families through personal resources from theprivate market. Children of poor families who cannot affordthese vaccines are at a disadvantage… The introduction ofnew vaccines in UIP is an approach to make vaccines accessibleto the poor and needy.” (5: p16) The committee proposesto include these new vaccines on the basis of availabilityand their use in other countries, but not on the basis of anyevidence from India. Once a vaccine is included in the nationalprogramme, the manufacturers secure a huge market in asingle stroke for years together, unlike in the case of othermedicines (1, 2). The introduction of more expensive vaccinessold by private manufactures in the public health systemrequires a transparent evaluation of the need for the vaccineand the health of the children in the country, and should notfocus solely on the viability of the vaccine industry (4, 6, 12).
The methods used by economically well-off nations to gaincontrol over poor countries by accessing their markets andcreating a demand for medical technologies, includingvaccines, irrespective of local needs, have been documentedextensively (1, 7, 9, 12). When a new product is being prepared, research is published to highlight the number of deaths causedin the country concerned due to the absence of that particularvaccine. The estimates are often outright exaggerated or reflectpoor research design. The limitations of such models have beenpointed out previously (12, 13, 14). In the next stage, after a marketpresence has been established, the equity argument is broughtup. Pressure is brought to bear on the government to bring thevaccine under the UIP with the argument that the well-to-doare protected and it is not equitable that the poor should gounprotected (12, 15). These methods are used to influence ourvaccine policy as well. The following two ethical issues are alsoto be considered:
- As vaccines are given to healthy populations, their safety and the need for them should be thoroughly assessed on the basis of various scientific parameters before they are introduced in a national programme (1, 2).
- The mere availability of a safe and efficacious /affordable vaccine cannot be a good enough justification for its universal use. Vaccines are not consumer goods and should not be advised unless the need for them is proven on the basis of scientific principles of public health(2).
Financial mechanism for newer vaccines
The vaccine policy also contains guidelines for a financialmechanism for the introduction, production and supply ofnewer vaccines in the UIP programme (5: p 10, 29). It comesout openly in favour of public-private partnership (PPP)and advance market commitment (AMC). Under AMC, thegovernment promises to buy a certain amount of vaccine at agiven price, even if the efficacy of the vaccine is poor or it hasa lower market price, thus guaranteeing the market before itsproduction. If we are being asked to make a long-term AMCbefore evaluating the utility of a vaccine, this policy needscareful scrutiny (2, 4).
The policy mentions several models for financing (5). “..inPakistan, where the rich kids pay a price for the [typhoid]vaccine that allows it to be subsidised to the poor kids. InBangladesh, the fishery industry finances the cholera vaccinefor the poor. Such models need to be studied and similar onesto be developed for India at least for some vaccines such aspneumococcal conjugate vaccine, rotavirus vaccine and HPVvaccine” (5: p 29). Here again, the policy does not discuss thesubject of these essential vaccines (6).
The policy suggests flexible governing and fundingmechanisms to support vaccine development in the PPP modebecause “It unifies the commitment of public sector to developproducts to improve health of the population with the privatesectors discipline and culture in business development andmarketing.”(5: p10). According to the policy, “industry mustbe provided a channel to voice its opinion, to be utilised inframing policy” (5: p11), which might allow the private partnersto influence policy for marketing their interests in the future (4, 6). It is even suggested that repositories in public sectorinstitutes and platforms in the Indian Institutes of Technologybe augmented to support the vaccine industry, to encourage it to manufacture vaccines whose risks may not justify their use (4, 6). An expert committee on vaccination had earlier cautionedthat all measures be taken to ensure that PPPs do not lead topublic spending and private profit (4). The proposed modelfor financing and pricing vaccines on the basis of retaining theinterests of the private vaccine industry may be ill-advised.
The Global Alliance for Vaccines and Immunization (GAVI),an international coalition of multiple funding agencies withvaccine manufacturers and non-government organisations,was formed in 1999. It decides on the global promotions ofvaccination (11). Pharmaceutical companies promote theiragendas by funding or otherwise gaining influence oversuch funding agencies (12, 15, 16). The dominance of the Billand Melinda Gates Foundation in GAVI makes it by far thelargest contributor to the vaccination programme. It hasbusiness interests in at least nine pharmaceutical majors (6).Representatives from private vaccine manufacturers andindustry-funded medical associations/academies must bespecifically prohibited to prevent conflicts of interest whileformulating vaccination policy (2, 6).
Together with prioritisation of private sector funding, thesepolicies may undermine the developing countries’ self-reliancein vaccine technologies, while jeopardising the sustainabilityof their vaccination programmes (17). The policy fails to lay anyroad map for the revival of public sector capacity in vaccines,except for suggesting that the public sector be managedalong the lines of the private sector (6). This aim of such apolicy seems to be to not have a policy and to utilise vaccinesindiscriminately, with epidemiology taking a back seat. Thepolicy is increasingly determined by the supply “push” by thepharmaceutical companies than the “pull” demand of provenpublic health needs (4, 7).
A critical review of the rotavirus and HPV vaccines
The introduction of newer vaccines into the immunisation programmes in India has been the subject of heated debate in recent years (12, 15). While a number of concerns have been identified, the one that receives precedence over the others is that the commercial interest of the vaccine manufacturing lobby often overrides public health interest (2, 4, 6, 18, 19, 20). The author had previously published review articles on the inclusion of the Hib and pneumococcal conjugate vaccines in the UIP (20, 21). This article attempts to make a critical review of the inclusion of the rotavirus and HPV vaccines in the UIP.
Rotavirus-disease problem
Rotavirus infection has a wide range of clinical manifestations,ranging from the absence of symptoms to severe diarrhoea (22). Virtually all children, in the developed as well asdeveloping countries, get infected with rotavirus diarrhoea bythe age of three (23). The incidence of the first infection, whichis most likely to be symptomatic, peaks between the agesof four to 23 months of age and the severity of the infectiondecreases with subsequent attacks. Milder cases are easilymanaged with oral rehydration at home and only the severecases and some moderate ones may require admission (24).
There are limited data regarding the morbidity and mortality ofrotavirus infection and no countrywide data are available yet.On the basis of an analysis of 40 studies in India between 1976and 1997, the median prevalence of rotavirus in hospitalisedcases of severe diarrhoea was estimated to be 18% (IQR 15%-23%) (25). According to the Indian Rotavirus Strain SurveillanceNetwork, rotavirus was found in approximately 39% ofhospitalised cases, the incidence being the highest amongchildren aged between 6 and 23 months <a class="reference" href="#twentysix" data-placement="top" data-trigger="hover" rel="tooltip" title="Kang G, Arora R, Chitambar SD, Deshpande J, Gupte MD, Kulkarni M, Naik TN, Mukherji D, Venkatasubramaniam S, Gentsch JR, Glass RI, Parashar UD; Indian Rotavirus Strain Surveillance Network. Multicenter hospitalbasedsurveillance of rotavirus disease and strains among Indian children aged (26).
In a WHO report which is widely quoted by many authors, itwas reported that the case fatality rate of rotavirus infectionwas 1 in 225, with most of the deaths occurring in the Indiansubcontinent (27). The report attributes these figures to studiesby the epidemiologist Roger Glass but does not specify whichstudies these are. The studies by Glass (25, 29) do not mentionmortality rates due to rotavirus from India. In a study fromthe West, the case fatality rate of rotavirus infection over a10-year period was 0.27% (28). Naturally, an early infectionimparts acquired immunity to subsequent rotavirus infection (30). A Mexican study reported that two infections in childrenprovided complete protection (31), and an Indian studyreported that three infections provided 79% protection (32).
Rotavirus vaccine
WHO has recommended the inclusion of the rotavirus vaccinein the national schedules of countries where the under-5mortality due to diarrhoeal diseases is &GreaterEqual10% (33). Currently, twovaccines are available. These are Rotarix (GlaxoSmithKline), amonovalent vaccine administered in two doses, and Rota Teq(Merck), a pentavalent vaccine administered in three doses,starting at 6-12 weeks of age. Both are given orally (33). Anindigenous vaccine, 116E (Bharat Biotech), which is based onhuman rotavirus of serotype G9P (11), is still under phase 2trials (33). The data from other developing countries showefficacy of Rotarix vaccine ranging from 17.6% in Mali to 61.2%in South Africa (34).There have been no efficacy trials of thelicensed rotavirus vaccines available in India (16,34,37). Thereis a definite gradient in the efficacy of these vaccines whendifferent regions of the world are compared-the highest is inthe USA and it is low in Asia (37, 38, 39, 40). A recent immunogenicitytrial in India for two doses of Rotatrix has shown a lowseroconversion rate of 58.3% (39).
The vaccine policy mentions that the 116 E rotavirus vaccinewas developed through effective collaboration betweenIndian and US academia, as well as partnership between theIndian vaccine industry and the Programme for AppropriateTechnology on Health (PATH). Safety and immunogenicitystudies of two orally administered human rotavirus vaccinecandidates, 116E and I321, were done in India (41).
For the evaluation of a vaccine for public health use, whatis more important than its efficacy are the absolute risk ofinfection, absolute risk reduction (difference between the riskof disease in the non-vaccinated and that in the vaccinated),the number needed to treat (NNT), and number needed toharm. These parameters /statistics also give a better idea of the cost-effectiveness of a vaccine (42). A multicentric randomisedcontrolled trial conducted in different Asian countries to testthe safety and efficacy of rotavirus vaccine RIX4414 (RotarixTM) yielded the following results (34). The experimental grouphad 5,263 children and the control group, 5,256. Two children(0.04%) among the experimental and 51 (0.97%) among thecontrol group developed severe rotavirus gastroenteritis in thetwo-year follow-up period, the efficacy of the vaccine being96.3% (95% CI 86.0- 99.6%). The actual risk difference forrotavirus infection among the control group was only 0.93%(0.97-0.04), which means that the clinical significance wasnegligible (34). The NNT to get one extra protection was 214per year. During the two years of follow-up, severe rotavirusinfection was not found to recur among infants from eithergroup. This suggests that acquired immunity develops afterthe first infection and the rotavirus vaccine is not needed forimmunity. Eight (14.9/10,000) and four (7.5/10,000) cases ofintussusception (an abdominal surgical problem in children)were reported among the vaccinees and control groups,respectively, and the risk was 7.4 /10,000 more amongvaccinees (34). The rotavirus vaccine was first withdrawn fromthe USA due to the risk of intussusception (35). Following thefirst dose, the risk was reported to be 1-2/100,000) (43). Thereare no published reports on the incidence or rates of acuteintussusception following rotavirus vaccination in India (16).Post-marketing surveillance data revealed that there had been13 cases of acute intussusceptions till December 2011, and twocases following RV5 during a five-month surveillance periodin India (16). A Cochrane database review reported that therotavirus vaccine failed to achieve mortality reduction amongchildren (36). The diminished immune response and lowerefficacy of oral vaccines in developing countries like India arewell known, especially among younger age groups, becauseof the greater interference of maternal antibodies (16, 34, 44).This has been shown to reduce the immunogenicity of oralpoliovirus vaccine earlier (34).
At present, the rotavirus vaccine is not manufacturedindigenously, though some Indian companies are tryingto manufacture it in collaboration with foreign companies.Compared to the cost of the primary vaccines included in theUIP, the cost of the routine rotavirus vaccine is prohibitive. Thecost of one dose is approximately Rs 770 and immunising allchildren in India with two doses would require Rs 1925 crore.Considering that the total budget for the UIP in India wasRs 1320 crore for the year 2011-12 (36), this option seemsimpossible. Though a model-based analysis regarding thepublic health impact of the rotavirus vaccine in India proved itto be cost-effective (22), a re-analysis showed that the absoluterisk reduction is only marginal or negligible for the most vitalof events, such as severe infections, death, outpatient visits andadmission to hospital (29).
In the industrialised countries of the West, general standardsof hygiene and improvement in sanitation led to the virtualextinction of diarrhoea due to bacterial and parasitic disease.This led to a proportional increase in the occurrence ofdiarrhoea due to rotavirus. Hence, the rotavirus vaccine may have been a public health priority for these countries.However, the same conditions do not apply to developingcountries like India, where the rotavirus strains are differentand 58% of rotavirus infections are associated with otherpathogens (29,36). Almost every child gets rotavirus infectionby the age of three years and there is little to worry aboutif dehydration can be managed promptly. Besides, earlyinfection with rotavirus affords good protection againstmoderate to severe diarrhoea. The following questions areboth scientifically and ethically relevant to guide our policyon rotavirus vaccination (29):
- What is the burden of rotavirus morbidity and mortality in the community setting in India?
- Is it more desirable to encourage the acquisition of natural immunity through mild rotavirus infection while focusing on improving the population’s nutritional status and the facilities for prompt management of severe dehydration, especially in the case of children between 2 and 24 months of age?
Many experts have opined that the recommendation for theinclusion of the rotavirus vaccine in the UIP in India should wait (16, 33). Its inclusion now would be a mistake (36).
HPV vaccine
Persistent HPV infection may lead to the development ofprecancerous lesions or severe adenocarcinoma in situ. Thesehave a high chance of progressing to squamous cell cancer oradenocarcinoma respectively, within an average of about 20years (45,46). There are more than 100 types of HPV, of whichat least 15 are oncogenic, and most of the HPV infections arecleared by the immune system (46). In some women, theinfection persists and some may develop precancerous cervicallesions, though the relationship between infection at a youngage and the development of cancer 20-40 years later is notclearly known (46).
In a recent study in India, a cohort of 31,488 women (age 30-59 years) were followed up over eight years. The absolute riskof cervical cancer was 2.5/10,000/year and HPV was detectedin only 10.3%, with the prevalence being almost similar acrossdifferent age groups (47). Even among the ‘HPV-positive’women, only 36.7% had lesions of cervical intra-epithelialneoplasia (CIN) grade 1 or higher. This raised questions aboutthe magnitude of the risk arising from HPV infection as far asthe development of (pre)cancerous lesions is concerned (47).According to the national cancer registry, the number of casesof cancer of the cervix is predicted to reach 113,138 by 2015and 123,291 in 2020 (48).
The current HPV vaccines target only two oncogenic strains:HPV-16 and HPV-18. These account for the majority of cancers (46). The currently licensed HPV vaccines-quadrivalent HPV 6,11, 16, 18 (Gardasil(r), Merck & Co., Inc., Whitehouse Station, NJUSA) and bivalent HPV 16, 18 (Cervarix ™, GlaxoSmithKlineBiologicals, Rixensart, Belgium)-are recommended for theage group of 9-26 years. Three doses of these injections arerequired to be administered in six months. There is a lack of conclusive data regarding the length of immunologicalprotection afforded by these vaccines (49).
In the case of HPV, no large field trials have been carried out inIndia and the data submitted before the licensing authoritiesfor marketing approval of Cervarix and Gardasil in the countryare not available in the public domain (18, 19, 49). Approval forthe HPV vaccines in India was based on two trials approvedby US regulatory agencies. The trials were conducted on smallsamples, that for Cervarix consisting of 354 “healthy Indianfemale subjects aged 18-35 years” and that for Gardasilconsisting of 110 “healthy females of 9-15 years of age” (18).The MOHFW, Indian Council of Medical Research, PATH and thestate governments of Andhra Pradesh and Gujarat conducteda ‘demonstration project’ to ascertain the feasibility of theintroduction of the vaccine among vulnerable girls in India. Inthese trials, three doses of the HPV vaccine were administeredto 16,000 girls of between 10 and 14 years of age in AndhraPradesh and Gujarat without monitoring adverse reactions Thisproject was widely criticised for its use of unethical practicesthat violated all scientific norms (18, 19, 49).
If we optimistically assume that the vaccine will prevent everycase of cervical cancer, the absolute risk reduction is 0.00025.The number needed to be vaccinated to prevent one death is4,000 and the cost per life saved can be calculated to be Rs 750lakh (47,50). Currently, there is no evidence on the number ofdoses of vaccines / frequency of boosters required for lifetimeprotection and the prevention of cervical cancer (18, 19). Thiscan be determined only through clinical trials and long-termfollow-up. No studies with relevant results have been published (51).
Currently, one dose of the vaccine costs Rs 3,000 (approximatelyUS$ 60). Thus, if every 10-year-old girl receives three shotsinitially (Rs 9,000) and needs a booster shot every five yearsover the next 40 years, amounting to eight shots in all (Rs24,000), the total cost would come to Rs 33,000 according tothe present estimates (49, 50).
Besides, information regarding the protection offered bythe HPV vaccine 30-40 years after the primary vaccinationis not available from anywhere in the world (53). No costeffectivenessanalyses have been carried out to determinewhether the proposed vaccination programme will result infewer deaths from cancer (54, 55). We must also consider thatHPV vaccination is not a substitute for screening for cervicalcancer. The expenditure required for screening of one womandoes not exceed Rs 80-250 and all women, including thosewho are vaccinated, are advised to continue to get regular Paptest screening and HPV testing done (18).
The adoption of more effective measures for the preventionand control of cervical cancer, including better hygiene, earlydetection through cytology-based screening programmesand the treatment of precancerous lesions, has substantiallyreduced deaths related to cervical cancer in the developednations (18, 19, 52).
India has several health priorities and the inclusion of theHPV vaccine in the government programme need not qualifyamong the highest in the list (19, 49). Given the unfavourablecost-effectiveness of the vaccine and the present healthexpenditure of the central and state governments, the cost ofintroducing the vaccine may not be justified (19, 49). In addition,there will be a need to cross hurdles of an ethical, religious,cultural and social nature as well as the vaccine is against asexually transmitted virus (52). The latest WHO committeeon HPV vaccine recommended that further monitoring wasneeded to learn about the impact of the vaccination onprecancerous lesions and cancer of the cervix as the currentevidence was insufficient to make decisions related to nationallevelusages (56).
The additional considerations which must be taken into account are (57): (i) the practical experience from HPV vaccination programmes worldwide has been limited; (ii) there is reluctance to accept a vaccine to prevent a sexually acquired infection that causes cancer only sometimes, and prevents infection only if it is completed before exposure; (iii) vaccination does not confer protection against all causes of cervical cancer; and (iv) an HPV vaccination programme has been rejected by some developed countries for the reasons discussed above (57).
GAVI is the major external funding agency for HPV in India andit has included HPV in its Advanced Market Commitment plan(18). This illustrates how the promotional practices of drugcompanies, pressure from powerful international organisations,and the co-option of India’s medical associations to uncriticallyendorse a vaccine are influencing public health priorities (18).Therefore, there are several factors that need to be given dueconsideration before recommending/ prescribing/using HPVvaccines on a large scale in India.
References
- Madhavi Y. Vaccine policy in India. PLoS Med. 2005 May;2(5):e127. Epub 2005 May 31.
- Puliyel J. Vaccine policy and advance market commitments. Econ Pol Wkly. 2011Nov 5;XLVI (44-5):18-19.
- Bhan A. Ethical considerations in developing a national vaccine policy. Indian J Med Res. 2010 Aug;132:226-7.
- Madhavi Y, Puliyel JM, Mathew JL, Raghuram N, Phadke A, Shiva M, Srinivasan S, Paul Y, Srivastava RN, Parthasarathy A, Gupta S, Ranga U, Lakshmi VV, Joshi N, Nath I, Gulhati CM, Chatterjee P, Jain A, Priya R, Dasgupta R, Sridhar S, Dabade G, Gopakumar KM, Abrol D, Santhosh MR, Srivastava S, Visalakshi S, Bhargava A, Sarojini NB, Sehgal D, Selvaraj S, Banerji D. Evidence-based National Vaccine Policy. Indian J Med Res. 2010 May;131:617-28.
- Ministry of Health and Family Welfare. National Vaccine Policy [Internet]. MoHFW, Government of India. New Delhi: 2001 Apr [cited 2013 May 12]:1-30. Available from: http://mohfw.nic.in/WriteReadData/ l892s/1084811197NATIONAL%20VACCINE%20POLICY%20BOOK.pdf
- Bajpai V, Saraya A. Agenda setting in vaccine policy and social relevance of the emerging vaccine technologies from public health perspective – part 1. Int J Med Public Health. 2012;2:7-15.
- Phadke A, Kale A. Some critical issues in the epidemiology of hepatitis-B in India. Indian J Gastroenterol. 2000;19 (Suppl 3):76-7.
- Dutta AG. What ails Delhi? Mid-day [Internet]. 2010 May 31 [cited 2012 Oct 13]. Available from: http://www.mid-day.com/news/2010/may/310510-Delhi-Diphtheria-826-cases-Pertussis-vaccine-scam.htm
- Madhavi Y. Vaccine PSUs: chronicle of an attenuation willfully caused. MFC Bull. 2008 Jun-Jul;329:1-7.
- Park K, editor. Park’s textbook of preventive and social medicine. 19th ed. Jabalpur: Banarsidas Bhanot Publishers; 2007:105-6.
- Indian Academy of Pediatrics, Committee on Immunization 2005-2006. 4th ed. Mumbai: IAP; 2007 Jan.
- Lone Z, Puliyel JM. Introducing pentavalent vaccine in the EPI of India: a counsel for caution. Indian J Med Res. 2010 Jul;132:1-3.
- Rao JV, Ganguly NK, Mehendale SM, Bollinger RC. India’s response to the HIV epidemic. Lancet. 2004;364:1296-7.
- Hardon A, Blume S. Shifts in global immunization goals (1984-2004): unfinished agendas and mixed results. Soc Sci Med. 2005;60:345-56.
- Puliyel JM, Madhavi Y. Vaccines: policy for public good or private profit? Indian J Med Res. 2008 Jan;127:1-3.
- Indian Academy of Pediatrics committee on immunization (IAPCOI). Consensus recommendations on immunization and IAP immunization timetable 2012. Indian Pediatr. 2012 Jul:49:549-63.
- Yamey G. Global Vaccine Initiative creates inequity; analysis concludes. BMJ. 2001;322:754.
- Sarojini NB, Srinivasan S, Madhavi Y, Srinivasan S, Shenoi A. The HPV vaccine: science, ethics and regulation. Econ Pol Wkly. 2010 Nov;XLV:27- 32.
- Ramanathan M, Varghese J. The HPV vaccine demonstration projects: we should wait, watch and learn. Indian J Med Ethics. 2010 Jan- Mar;7(1):43-5.
- Jayakrishnan T. Newer vaccines in the Universal Immunisation Programme. Indian J Med Ethics. 2011 Apr-Jun;2(2):107-12
- Jayakrishnan T. Pentavalent vaccine in UIP – a review. Indian Pub Health Assoc Bull (Kerala). 2011;2:7-9.
- Rose J, Hawthorn RL, Watts B, Singer ME. Public health impact and cost effectiveness of mass vaccination with live attenuated human rotavirus vaccine (RIX4414) in India: model based analysis. BMJ. 2009;339:b3653 doi:10.1136/bmj.b3653.
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, Jiang B, Gentsch JR. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368(9532):323-32.
- Parashar UD, Glass R I. Rotavirus in viral gastroenteritis. In: Harrison’s Principles of Internal Medicine, 16th edition, Kasper DL, Braun-wald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. New York: McGraw- Hill, Medical Publishing Division; 2005: p 1141-2.
- Jain V, Parashar UD, Glass RI, Bhan MK. Epidemiology of rotavirus in India. Indian J Pediatr. 2001;68:855-62.
- Kang G, Arora R, Chitambar SD, Deshpande J, Gupte MD, Kulkarni M, Naik TN, Mukherji D, Venkatasubramaniam S, Gentsch JR, Glass RI, Parashar UD; Indian Rotavirus Strain Surveillance Network. Multicenter hospitalbased surveillance of rotavirus disease and strains among Indian children aged 5 years. J Infect Dis. 2009 Nov 1;200 Suppl.1:S147-53.
- World Health Organization. Report of the meeting on future directions for Rotavirus Vaccine Research in Developing Countries[Internet]. Geneva 9-11 Feb 2000. Geneva: Department of Vaccines and Biologicals, WHO;2000 [cited 2013 May 12]. Available from: http://whqlibdoc.who.int/hq/2000/WHO_V&B_00.23.pdf
- Gil-Prieto R, San Martín M, de Andrés A L, Alvaro-Meca A, González A, de Miguel AG. Hospital acquired rotavirus infections in Spain over a tenyear period (1998-2007). Hum Vaccin. 2009 Nov;5(11):748-53.
- Bajpai V, Saraya A. Agenda setting in vaccine policy and social relevance of the emerging vaccine technologies from public health perspective – Part I1. Int J Med Public Health. 2012;2:16-25.
- Phua KB, Emmanuel SC, Goh P, Quak SH, Lee BW, Han HH, Ward RL, Bernstein DI, De Vos B, Bock HL. A rotavirus vaccine for infants: the Asian experience. Ann Acad Med Singapore. 2006 Jan;35(1):38-44.
- Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter- Campbell S, Glass RI, Estes MK, Pickering LK, Ruiz-Palacios GM. Rotavirus infection in infants as protection against subsequent infections. New Engl J Med. 1996 Oct 3;335(14):1022- 8.
- Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, Jaffar S, Gomara MI, Gray JJ, Brown DW, Desselberger U, Crawford SE, John J, Babji S, Estes MK, Kang G. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med. 2011 Jul 28;365(4):337- 46.
- Taneja DK, Malik A. Burden of rotavirus in India–is rotavirus vaccine an answer to it? Indian J Pub Health Assoc. 2012 Jan-Mar;56:17-21.
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, Lee BW, Teoh YL, Tang H, Boudville I, Oostvogels LC, Suryakiran PV, Smolenov IV, Han HH, Bock HL. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine. 2009 Oct 9;27(43): 5936-41.
- Rose J, Parashar UD. Should India launch a national immunization programme against rotavirus. Yes. Head to head. BMJ. 2012 Nov 30:345:e7818.doi:10.1136/Bmj.e7818.
- Puliyel JM, Mathew J. Should India launch a national immunization programme against rotavirus. No. Head to head. BMJ. 2012:345e 7832. doi:10.1136/Bmj.e7832.
- World Health Organization. Rotavirus vaccines: an update. Wkly Epidemiol Rec. 2009;84:533-40.
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007 Nov 24;370(9601):1757-63.
- Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, Datta SK, Suryakiran PV, Delem A, Han HH, Bock HL. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin. 2009 Jun;5(6):414-19.
- PATH, Norwegian Institute of Public Health, CDC and WHO. Proceedings from the 8th International Rotavirus Symposium. Organized by PATH, Norwegian Institute for Public Health, CDC and WHO, Istanbul, Turkey, June 3-4, 2008.
- Bhandari N, Sharma P, Glass RI, Ray P, Greenberg H, Taneja S, Saksena M, Rao CD, Gentsch JR, Parashar U, Maldonado Y, Ward RL, Bhan MK. Safety and immunogenicity of two live attenuated human rotavirus vaccine candidates, 116E and I321, in infants: results of a randomised controlled trial. Vaccine. 2006 Jul 26;24:5817-23. Epub 2006 May 12.
- Spitalnic S. Risk assessment: relative risk and absolute risk reduction. Hospital Physician. 2005 Oct;10:43-6.
- No authors listed. Rotavirus vaccine and intussusception: report from an expert consultation. Wkly Epidemiol Rec. 2011 Jul 22;86(30):317-24.
- Paul Y. Oral polio vaccines and their role in polio eradication in India. Expert Rev Vaccines. 2009;8:35-41.
- World Health Organization. Human Papillomavirus Vaccines WHO position paper. Wkly Epidemiol Rec. [Internet]. 2009 Apr 10[cited 2012 Nov 29];15:118-32. Available from: http://www.who.int/wer/2009/wer8415.pdf
- Haug C. The risks and benefits of HPV vaccination JAMA. 2009;302:795-6.
- Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, Hingmire S, Malvi SG, Thorat R, Kothari A, Chinoy R, Kelkar R, Kane S, Desai S, Keskar VR, Rajeshwarkar R, Panse N, Dinshaw KA.. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385-94.
- Time trend of cancer incidence rate 1982-2005.National Cancer registry programme [Internet] ICMR. 2004 Apr[cited 2012 Dec 23]. Available from: http://www.icmr.in/ncrp/cancer_reg.htm
- Anjali, Sarojini, Sama, with inputs from various individuals and organisations. Memorandum: Concerns around the human papilloma virus (HPV) vaccine. Indian J Med Ethics. 2010 Jan-Mar; 7(1):37-40.
- Aneja H, Puliyel JM. Selling vaccines: deciding on who can afford HPV. Indian Pediatr. 2009;46:647.
- Sawaya GF, Smith-McCune K. HPV vaccination: more answers, more questions. N Engl J Med. 2007;356:1991-3.
- Somasundaram K. HPV vaccine: end to women’s major health problem? Indian J Med Res. 2008 Jun;127(6):511-13.
- Mathew JL. HPV vaccine in the Indian context. Indian Pediatr.2009 July;46(7);644-6.
- Choudhury P. Preventing cervical cancer: pediatrician’s role. Indian Pediatr. 2009 Mar;46(3):201-3.
- National Board of Health. Reduction in the risk of cervical cancer by vaccination against human papilloma virus (HPV) – a health technology assessment. Copenhagen: National Board of Health, Danish Centre for Health Technology Assessment, 2007[cited 2013 May 12]; 9:1-14. Available from: www.dacehta.dk
- World Health Organization. Meeting of WHO human papilloma virus advisory committee – April 2010. Wkly Epidemiol Rec. 2011May 27;86(22):221-32.
- Comeau P. Debate begins over public funding for HPV vaccine. CMAJ. 2007;176:913-14.
