ARTICLE
Knowledge and perceptions of research participants in Nigeria about clinical trials
Babatunde Adewale, Lizette Schoeman, Theresa Roussouw
DOI: https://doi.org/10.20529/IJME.2015.053
Published online: August 25, 2015
Abstract
Benchmarks of ethical research in developing countries stipulate collaborative partnership, which necessitates the involvement of research participants and taking cognizance of their opinions in decisions regarding research activities. Little data regarding participants’ perceptions about research activities exists in the developing world. This study assessed the knowledge and perceptions of research participants in Nigeria about clinical trials. A validated semi-structured questionnaire was used in a cross-sectional survey. Data were analysed using SPSS version 17. Seventy-five respondents (70.7% females, 29.3% males) with a mean age of 36.5±10.3 years, enrolled in an efficacy and safety study of Artequin in Ikorodu LGA, Nigeria, participated in the survey. Most of them (64%) had secondary education while 6.7% were illiterate. Only 5 (6.7%) had previously participated in a clinical trial. The majority of respondents (70.7%) did not know how medicines are determined to be safe and none knew how new drugs are tested. While only 10(13.3%) respondents felt that people were well treated during clinical trials, only two knew of someone who had been harmed because of participation and only one respondent could report on the type of harm experienced by the participant. The majority (86.7%) did not know if people were well treated or abused or whether people’s rights were protected during clinical trials (84%). Despite being enrolled in a clinical trial, participants have limited knowledge about such trials. This lack of knowledge might impact the quality of informed consent provided. If true collaboration is to be achieved in developing world settings, the community in general, and trial participants in particular, should be educated about the basic principles of research.
Introduction
There has been increased funding for research on diseases affecting the world’s poor, as researchers in public and private sectors continue to strive to achieve the goal of developing improved diagnostic tools, prevention strategies and interventions to counter the debilitating impact of diseases (1). The benchmarks of ethical research in developing countries stipulate collaborative partnership, which necessitates involvement of research participants in the research endeavour, for instance by taking their opinions seriously regarding research activities (2). This study assessed the knowledge and perceptions of research participants in Nigeria about clinical trials, specifically about how new drugs come to market, how drugs are tested, and how research participants are treated. This was part of a larger study on the voluntariness and understanding of informed consent in a clinical trial, issues which, according to Molyneux et al (3), have received little attention in the developing world.
Methods
The study design and questionnaire were approved by the ethics committees of the University of KwaZulu-Natal (Approval Number: HSS/0247/2010 M) and the Nigerian Institute of Medical Research. Approval was obtained before the commencement of the project.
The questionnaire was a validated, semi-structured instrument that had been adapted from Barsdorf and Wassenaar (4). It was administered to a cross-section of participants enrolled in a hospital-based efficacy and safety study of an anti-malarial drug in a rural community in Ikorodu local government area in Nigeria. Permission to approach trial participants was obtained from the principal investigator of the trial as well as from the hospital administration. All participants gave informed consent before administration of the questionnaire.
Inclusion criteria
Adults, older than 18 years of age and resident in the community, who had consented to participate in the antimalaria trial (either on behalf of themselves or on behalf of a child under the age of 5 years) and were willing and able to give informed consent for participating in this study. After an appointment had been scheduled telephonically, a trained field assistant and the investigator interviewed individual participants in a private space in their homes. Questions were asked in the local language and responses were written after ascertaining that the participants understood the question asked.
Sample size
There were 360 participants in the anti-malaria trial. Assuming a prevalence of involuntariness and misunderstanding of 50% (5), it was calculated that a sample size of 75 was adequate to meet the study end-points with statistical power of 80% and level of significance of 95%. Participants were selected by means of systematic random sampling: the total number of participants in the malaria trial was divided by the required sample size to obtain the sampling fraction (360/75=~5). The sampling fraction was then used as the constant difference between participants, meaning that every fifth participant in the malarial trial was eligible for selection.
Data analysis
Completed questionnaires were pre-cleaned, coded and analysed using SPSS for windows version 17 (SPSS inc., 1999). Basic descriptive analysis (such as means, proportions, frequencies and range) and limited analysis (correlation, chisquare and Fisher Exact) of the possible association between the predictor variables and the outcome variables were done.
Results
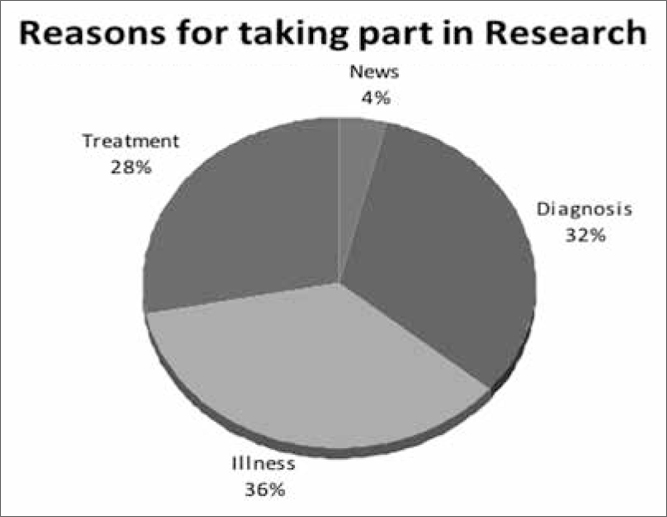
All the 75 invited persons gave informed consent to participate in the study. Their mean age was 36.5±10.3 years (range 18–57 years). The majority, ie 53 (70.7%), of the respondents were females and 64 (85.3%)were married. Their educational background ranged from no education (6.7%), to primary (14.7%), secondary (64%) and tertiary (14.7%) education. Just over half (53.3%) were involved in trading as a profession. Their reasons for participation varied, as shown in Figure 1, with the majority participating because they were ill and/ or expected a diagnosis and treatment of their symptoms. None of the respondents received additional information from other sources about the clinical trial before participation apart from 10 (15.6%) women among the married respondents who sought permission from the heads of their household. Only 5 (6.7%) had previous experience of clinical trials.
Despite the fact that these respondents were participating in a clinical drug trial, the majority (70.7%) did not have any idea how new medication is tested and determined to be safe or effective. Thirteen participants (19.7%) said that efficacy and safety were determined when medicines are used by people who are ill, 3 (4.0%) associated this aspect with research/ testing (Table 1) while only 4 (5.3%) knew that medicines are first tested in laboratory animals before they are tested in humans. Five (6.7%) participants knew that medicines found to be safe in animals needed to be tested in humans before being sold to the public. No significant relationship was found between the age of respondents and knowledge of preclinical testing of medicines in laboratory animals (p=0.616) or knowledge that new medicines found to be safe in animals needed to be tested in humans before being sold to the public (p=0.344) (Table 2).
Fifty-nine (79.70%) respondents believed that the people who should be involved in medical research are sick people, followed by 15 respondents (20.3%) who believed that people involved in research should be volunteers. These responses were independent of age (p=0.87), gender (p=0.078), religion (p=0.75),and marital status (p=0.414) (Table 3).The majority of respondents, ie 73 (97.3%), thought that it was fair to use human beings for research, 1 (1.3%) thought it was not fair, while 1 (1.3%) did not know whether it was fair or not. The major reason offered for the necessity of human research was that humans were the end users – 70 (94.6%). One respondent felt that animals should be used for research instead of human beings.
Only 10 (13.3%) respondents felt people were treated well during drug trials because their ailments were treated, medicines were efficacious and beneficial, and normality was restored, while the rest, ie 65 (86.7%), did not know if people were treated well or not. One (1.3%) respondent thought that people’s rights are abused since they are neglected when medicines are tested on them. Eleven (14.7%) participants believed that people’s rights are protected while 63 (84.0%) were not sure. Two (2.7%) respondents knew of people who had been harmed or disadvantaged because medicines were tested on them. However, only one of these respondents could describe the type of harm or disadvantage experienced by the clinical trial participant.
| Table 1: How safety and efficacy of medicine are determined | |
| Mode of determination | n (%) |
| Research/testing | 3 (4.0) |
| Read package insert | 5 (6.75) |
| Tradition/word of mouth/reputation | 1 (1.3) |
| When used | 13 (19.7) |
| Don’t know | 53 (70.7) |
| Total | 75 |
| Table 2: Knowledge of testing medicine | |||||
| Age (years) | Yes n (%) | No n (%) | Total (n) | p-value (Fisher Exact) | |
| Testing medicine first in laboratory animals | <35
≥ 35 Total |
2 (7.4)
2 (4.2) 4 (5.3) |
25 (92.6)
46 (95.8) 71 (94.7) |
27
48 75 |
0.616 |
| Testing medicine in humans | <35
≥ 35 Total |
3 (11.1)
2 (4.2) 5 (6.7) |
24(88.9)
46 (95.8) 70 (93.3) |
27
48 75 |
0.344 |
| Table 3: Participants’ opinion on who should be involved in research | ||||
| Characteristic | Type of study participant | Total | Statistics | |
| Illness (n=59) | Volunteer (n=15) | |||
| Age (years)
<35 ≥35 |
21 (35.6) 38 (64.4) |
5 (33.3) 10 (66.7) |
26 (35.1) 48 (64.9) |
X2=0.026 p=0.87 |
| Gender
Male Female |
14 (23.7) 45(76.3) |
7 (41.1) 8 (58.9) |
21 (28.4) 53 (71.6) |
X2=3.1 p=0.078 |
| Religion
Christianity Islam |
16 (27.1) 43 (72.9) |
5 (33.3) 10 (66.7) |
21 (28.4) 53 (71.6) |
p=0.75* |
| Marital status
Married Single |
52 (88.1) 7 (11.9) |
12 (80.0) 3 (20.0) |
64 (86.5) 10 (13.5) |
p = 0.414* |
Discussion and conclusion
Our results show that participants did not have adequate knowledge about what constitutes a clinical trial. It seems that the majority consented to take part in the trial with the objective of receiving better medical care by accessing free treatment and laboratory investigations. The inherent danger of this motivation for participation is the possibility of overestimating the potential benefits that may be obtained or the proven nature of the study intervention. Failure to recognise the primary purpose of the trial, namely the generation of new knowledge (6), may lead to disappointment if expectations, especially treatment expectations, are not met. This might negatively affect participants’ perceptions of clinical trials, which may impact negatively on future participation in clinical trials. Though a few participants consulted people of authority, such as heads of households, about their participation in the trial, none sought a second professional opinion. This is unlike trials in developed countries where participants are more likely to discuss the risks and benefits of a trial with other professionals before giving consent (5). This might be due to the fact that participants in developing countries are less knowledgeable about clinical trials and may be swayed by what they perceive to be an opportunity to have their illness treated.
Patients and participants in clinical trials in developing countries generally lack the agency to negotiate their healthcare and are dependent on solutions from the biomedical community. This dependence is exacerbated by the dire need for safe and effective approaches to the treatment of diseases in the developed world. It seems that the knowledge of participants in a clinical trial about the process of biomedical research is very limited and often even plainly incorrect. This could hamper the process of informed consent, which is a vital step in the ethical implementation of clinical trials (7). These findings reinforce the need to raise awareness through health-related education about the research process so that participants can make informed decisions about participation in clinical trials.These findings also highlight the important role of investigators in assisting participants to attain adequate understanding of the contents of the information sheet (8).
Acknowledgements
This project was supported by Award Number R25TW001599 from the Fogarty International Center to the South African Research Ethics Training Initiative (SARETI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or National Institutes of Health.
References
- Udrea G, Dumitrescu B, Purcarea M, Balan I, Rezus E, Deculescu D. Patients’ perspectives and motivators to participate in clinical trials with novel therapies for rheumatoid arthritis. J Med Life. 2009;2: 227-31.
- Emanuel EJ, Wendler D, Killen J, Grady C. What makes clinical research in developing countries ethical? The benchmarks of ethical research. J Infect Dis. 2004;189: 930-7.
- Molyneux CS, Wassenaar DR, Peshu N, Marsh K. Even if they ask you to stand by a tree all day, you will have to do it (laughter)…: community voices on the notion and practice of informed consent for biomedical research in developing countries. Soc Sci Med. 2005;61:443-54.
- Barsdorf NW, Wassenaar DR. Racial differences in public perceptions of voluntariness of medical research participants in South Africa. Soc Sci Med. 2005;60:1087-98.
- Bhansali S, Shafiq N, Malhotra S, Pandhi P, Singh I, Venkateshan SP, Siddhu S, Sharma YP, Talwar KK. Evaluation of the ability of clinical research participants to comprehend informconsent form. Contemp Clin Trials. 2009;30: 427-30.
- Hoffner B, Bauer-Wu S, Hitchcock-Bryan S, Powell M, Wolanski A, Joffe S. Entering a clinical trial: is it right for you? A randomized study of The Clinical Trials Video and its impact on informed consent process. Cancer. 2012;118:1877-83.
- Perrey C, Wassenaar D, Gilchrist S, Ivanoff B. Ethical issues in medical research in the developing world: areport on a meeting organised by Fondation Mérieux. Dev World Bioeth. 2009;9: 88-96.
- Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593-601.
