ARTICLE
Audiovisual informed consent process in vaccine trials: Experience from North India
Madhu Gupta, Jaya Prasad Tripathy, Sanjay Verma
Published online: May 28, 2018
DOI: https://doi.org/10.20529/IJME.2018.043
Abstract
Audiovisual (AV) recording of the informed consent process in a clinical or vaccine trial to document the consent process of participants (especially from vulnerable populations), ensures preservation of their rights and well-being. This paper describes the AV consent process during a phase III rotavirus vaccine trial among healthy infants in Chandigarh and examines its effects. Out of 155 parents/guardians of participating infants who were contacted to be a part of the study, 50 were reluctant to participate in the study trial (not necessarily in the AV consenting process). Among 105 parents/guardians of participating infants who expressed initial willingness to participate in the trial, all agreed to undergo the AV consenting process; and 100 finally consented to participate and were enrolled in the study. So, the participation rate was 64.5% (100/155) among those who were contacted, and 95.2% (100/105) among those who underwent the AV consenting process. AV recordings of these 100 patient representatives were transcribed and later translated into English for a thematic analysis of the text. A total of 105 queries were raised by 55 participants. All queries were patiently listened to and addressed, allaying most fears, especially those related to adverse events following intervention. The AV process ensured transparency and accountability of the investigators, responsive referral mechanism in case of adverse events, building an initial rapport with the participant, complete vaccination of the trial subjects, and provision for free private care consultation depending upon the willingness of the parents. These benefits of the AV consent process might have led to a higher participation rate.
Introduction
In the past decade, serious concerns have been raised regarding the lack of adherence to ethical conduct during recruitment of vulnerable subjects in clinical trials in India (1). Although it is mandatory under Schedule Y of the Drugs and Cosmetics Rules, 1945 to obtain freely given informed, written consent from a study subject before enrolment in a clinical trial, there have been several complaints about the misuse of this provision by research institutes, pharma companies, and clinical research organisations who are engaged in clinical trials (2). It was reported that “informed consent” was frequently taken from participating subjects without informing them about the benefits, and importantly, the harmful side effects of the investigational product in the trial (3). It is assumed that the subject will not be able to understand the technicalities involved in the trial; hence, only the most essential information is provided to the subject and “informed” consent is said to be taken (3).
Taking serious note of this, the Office of the Drugs Controller General of India (DCGI), with approval from the Ministry of Health and Family Welfare, has made audiovisual (AV) recording of the informed consent process of each trial participant essential, in addition to written informed consent (4). The AV recording and related documentation should be preserved safely and confidentially and secured with password-protected software after the completion/termination of the study for a period of at least five years—if it is not possible to maintain the same permanently. These directives by the DCGI have not only made the pharmaceutical companies more wary of the situation but also cautioned investigators who will be involved in conducting the trials, which may influence their decision regarding participating in / conducting such trials.
Earlier studies have shown that more than one-third of study subjects, ranging from 30% to 50%, refused to give consent for AV recording of consent. Lack of interest in recording, dislike of being recorded, discomfort with and suspicion of being videotaped, shyness, and hesitancy were common reasons for refusal cited by the study subjects (5, 6, 7, 8).
This paper describes the experience of the authors with obtaining AV consent from subjects’ (healthy infants) parents in a phase III rotavirus vaccine trial so as to document the process involved in AV consenting, including the queries raised by the participants and how the queries were addressed. It also compares the sociodemographic differences between those who raise questions and those who do not. These findings will be useful for investigators/researchers in following the AV consent process while conducting trials and may allay their apprehensions regarding an increase in participant refusal rate.
Methods
Study sample
AV consent was taken from the parents / guardians / legally accepted representatives (LARs) of healthy infants aged 6 to 8 weeks in a phase III multicentre rotavirus vaccine trial in India by the investigators (authors) themselves (9). This trial was sponsored by Shantha Biotech Limited (a Sanofi Company). In this paper, we discuss the experience of AV consenting at one of the trial sites, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. The AV informed consent process was used for recruiting 100 healthy infants into this trial at this site in accordance with the new rules laid down by the DCGI.
Ethical considerations
The Institute Ethics Committee of PGIMER had approved the main vaccine trial (letter no. PGI/IEC/2014/516, dated 21/8/2014), including the informed consent form. Since no fresh data was collected for this study, and only a retrospective analysis of data already collected was conducted, the ethics committee was notified (author notification letter no. SPH/18/1016, dated 1/5/18). The committee responded vide letter no. PGI/IEC/2018/000642, dated 3/5/2018. The committee in its letter confirmed that fresh approval was not required and permitted the authors to proceed.
Setting up of AV consenting
A portion of a room was dedicated for AV recording through artificially erected barriers to maintain participant confidentiality and eliminate outside noise in the recording. Recording was done using a webcam (C9210 Logitech HD Pro) mounted on a desktop computer. The investigators were trained in carrying out AV recording by experts sent by the sponsors, by means of a dummy video on how to obtain AV consent. As the first step, consent to record audio and video for AV consent gathering was obtained from the parents/guardians/LARs (hereafter, “participants”). Only if they agreed to being audio- and videographed was the AV informed consent obtained.
The major content of the AV consent discussion is presented in Table 1. The written informed consent form was provided in English, Hindi, and Punjabi. A structured AV consent module was followed to ensure that all the components of informed consent were covered. All the components were given equal importance while explaining them to the prospective participants. Adequate time was given for the discussion and settling of queries. Information was given individually in the language that each participant was most conversant in. In case of participants who were illiterate, AV consenting was done in the presence of an impartial witness. Impartial witnesses were independent of the trial and included attendants of other patients in the study hospital, community representatives such as a local leader, and so on. The impartial witnesses attended the AV consent process and read the informed consent form and any information sheet supplied to the participant. The informed consent forms were signed by the participant / impartial witness under AV recording, and a copy of the form was given to each participant for their records. The study investigators (authors) trained for the AV consenting process remained throughout the trial. The duration of the AV consenting process varied from 30 to 45 minutes. The infants (subjects) had accompanied the participants to the site.
| Table 1: Content discussed in the audiovisual informed consent process | |
| Content | Description |
| Introduction | Introduction of investigator and the participant; welcoming the participant into the trial; inviting queries; building rapport. |
| Thanking the participant for agreeing to the AV recording | Building rapport; making the participant comfortable. |
| Background and rationale of the study | Explanation of rotavirus infection among infants and children; severity; incidence; symptoms; role of the rotavirus vaccine. |
| Number of children participating in the study | Total sample size; sample to be recruited from each centre in a multicentre trial. |
| Purpose of the study | Explanation given regarding the objective of the study: to evaluate the ability of the new rotavirus vaccine (investigational product) under this trial to produce antibodies that may have the ability to prevent infection from rotavirus compared to an approved vaccine. |
| Length of participation |
Information on how long the study participants are required to be part of the study. In this study, participants were told that their participation would last for 12–15 weeks—until completion of 28 days after the third dose. |
| Study procedures |
No undue influence to participate; ample opportunity to enquire about the details the study; signing the informed consent form; evaluation of the child as per the inclusion and exclusion criteria; recruitment into the trial. |
| Description of the intervention |
Details of the vaccines to be administered; blood sampling; stool sampling; home visits; diary cards for monitoring of adverse reactions and other health events. |
| Study visits | Timing of visits; activities during each visit; home visits. |
| Risks and side effects of participation |
Minor and major side effects of each vaccine; information on intussusception; referral mechanism in case of any adverse reaction. |
| Payment for taking part in the study |
Explanation regarding there being no cash payment for participation in the study, except for travel costs to visit the study site and any medical cost incurred during the study period. |
| Possible benefits for taking part in the study | No direct benefit; benefit of health examination and general health discussions with the study doctor. |
| Possible benefits to others | Benefit to the community. |
| Other available treatments |
Participants’ freedom to choose or purchase available licensed vaccines against the targeted disease from the market, if they do not want to participate in the trial. |
| Voluntary nature of participation | Voluntary participation; no undue influence. |
| Compensation and treatment | Participants explained about: provision of stipend according to the local practice to compensate for the travel required to visit the study site; compensation in case of injury or death during trial by the sponsors [as per Rule 122-DAB of GSR 53(E), Gazette Notification, Government of India]*; facilitation of treatment in a referral/higher centre if needed along with reimbursement of the cost of the treatment; and insurance coverage for all the subjects was provided by the sponsors to pay damages in respect of injury caused by or arising due to participation by the subjects in the trial for one year |
| Responsibilities of the participant |
Follow the instructions; return to the clinic for scheduled visits; complete the diary card; promptly report to the staff any unexpected or serious health events; inform the study doctor of any medications the child takes during the study. |
| Termination of participation |
Participants informed about discontinuation in the study for failure to follow instructions in the study protocol, such as when to return for the next visit, or if sponsors or regulatory bodies decided to stop the study. |
| Confidentiality | Child’s participation kept confidential; auditors, ethics committee, regulatory authorities granted access to medical records. |
| Right to refuse or withdraw | Participants informed of their freedom to withdraw participation in the study at any time, with no effect on the treatment received for any ailment in the study hospital. |
| Dissemination of new information | Participants promised any new information about the vaccine. |
| * Central Drugs Standard Control Organization. Drugs and Cosmetics Rules 1945, Amendment Notification. New Delhi: Ministry of Health and Welfare, Government of India; 2013 Jan 30. The Gazette of India; Extraordinary Part II, Section 3, Sub-section (i), G.S.R. 53(E). Available from: http://www.cdsco.nic.in/writereaddata/GSR%2053(E).pdf. Accessed on 28 February 2018. | |
Data source and analysis
Data were extracted from the archived trial database. AV recordings were first transcribed in the local language (Hindi/Punjabi) and then translated into English by the study nurse, a postgraduate in sociology. Since this was qualitative data, thematic analysis of the translated text was done manually by the study investigators. The obtaining of AV consent, queries raised by the participants, and the responses of the investigators were also documented. Sociodemographic characteristics of the participants were summarised using percentages.
Results
Out of the 155 participants who were contacted to be a part of the study, 50 were reluctant to participate in the trial study (not necessarily the AV consent process) and refused. The remaining 105 participants had expressed their initial willingness to be a part of the study and undergo the AV consent process. However, they did not have much idea about the trial. Out of these 105 participants who provided AV consent, 100 participants finally agreed to participate in the trial. So, participation rate was 64.5% (100/155) among those who were contacted and 95.2% (100/105) among those who underwent the AV consenting process. The flow chart showing the enrolment status is presented in Figure 1. Of the enrollees, more than half (58%) of the infants were males, three-fourth (76%) coming from rural areas with annual household income ranging between INR 12,000 and INR 4,80,000. The infant was accompanied by father and mother in 65% of cases, only father in 4%, and only mother in 31%; a majority of the mothers were housewives (57%). Nearly 62% of the participants were educated up to middle school level. Table 1 details the type of information communicated in the AV informed content process and provides detailed descriptions on each subhead.
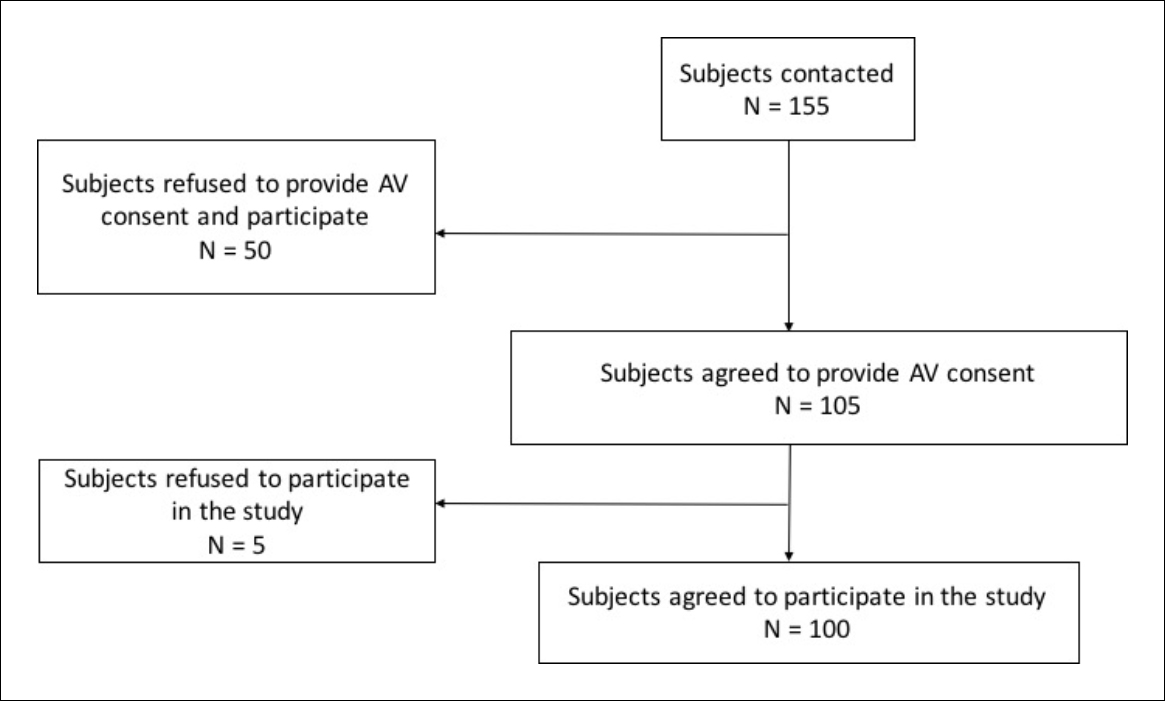
Out of 100, only 55 (55%) participants had any query. A total of 105 queries were raised, with 27 (27%) participants raising only one query each, 12 (12%) raising two queries, 12 (12%) raising three queries, two (2%) raising four queries, and two (2%) raising five queries each. The majority of the 105 queries were around where to consult in case of any emergency (22; 21%), risks to the baby as a result of the vaccine (20; 19%), vaccination schedule and any change in the schedule (15; 14%), and what would happen if they had to go out of the city (10; 9.5%). Some of them also had questions about the financial implications of participating in the study, whether they could avail private hospital care, about the study procedures, and what the results of the trial were until then. Some of the participants seemed intimidated in the beginning, but once the AV consent process was explained to them and they started to participate in it, they became more relaxed and comfortable. They were scared of participating in the study because of the fear of having a major problem in the future.
Out of 31 women who had consented, 19 (61.3%) women had asked questions. Only women asked the questions “Where to go if the baby becomes sick or has a problem after vaccination,” “Why are you taking the video?” “Can I call you if the baby has a problem?” “Will you give DPT vaccine too?” “Is it safe?”, “Will you draw blood a second time as well?” “What if we have to travel to the village in between?” “Can we take treatment privately?” “Will you give medicines also?”, and so on. It seemed that only when they were satisfied that their child would be taken care of completely during the study period did they give their consent for participation in the study. Among these 19 women, consent from two women was obtained in the presence of an impartial witness as they had studied only up to or less than primary level. These women consulted their husband/neighbour/friend before giving final consent. The remaining women (17/19) had all studied beyond or up to Class 8 and could read and understand Hindi; this included one graduate and two postgraduate mothers. Overall, the proportion of women who had studied up to or beyond Class 8 was higher among those who had asked questions (17/19; 89.5%), as compared to those who had not (7/12; 58.3%). The more educated women were more active in questioning the investigators and presumably could take a more informed decision than the less educated women. The proportions of women belonging to rural areas who had asked questions (16/19; 84.2%) and who did not (10/12; 83.3%) were almost similar.
It was observed that rural women asked more questions on the safety of the child, such as, “What to do in case there is a problem after vaccinating the child?” “Can I contact you in case of problems with the child later?” and “Will the child be eligible if we have to travel in between the study?” and regarding the waiting period following vaccination, such as “Will we have to wait for half an hour after vaccination?” They were also more apprehensive about the video recordings and blood sampling and had asked questions such as “Why are you making a video?” and “Will you draw the blood sample again?”
The urban mothers wanted more explanations on the trial being done in India, such as “Where and among how many children is this study going on?” They had asked more informed questions, such as, “What type of oral vaccine is the rotavirus vaccine?” “Are there side effects other than those you have mentioned or any severity?” “The baby is on calcium and iron supplementation; will these need to be stopped?” “May I call you at night?” “Can I take your phone number?” “Are any fees to be paid by the parents to participate in the study?” “Will you give the vaccine which is due at 45 days of birth of the child?” and so on.
The proportion of only mothers (19/31; 61.3%) who were asking questions was higher, as compared to the mother-and-father (35/65; 53.8%) or only-fathers (1/4; 25%) groups (Table 2). The most common questions asked when both mother and father were present during the consenting process were almost similar to those of the only-mothers group and were related to the type and safety of the study vaccine, such as, “What type of vaccine is this?” “What are the side effects?” “What to do if there is a problem?” “Where to take the child at night?” “Are doctors available at night?” “Who will be responsible for the child?” and “Who will give medicine if the child gets sick during the study?” Other frequently asked questions were related to the eligibility of the child to participate in the study in case the family had to travel during the study period. In the only-mothers group, the frequency of blood sampling was also enquired about. However, information about the study was asked for more often when both the parents were present; for example, “What are the other sites where this study is being conducted in India?” “What will be investigated in the blood sample of the child?” “Will the informed consent form and information sheet be available to read at home?” “What are other side effects that you may not have mentioned?” “Can treatment from a private health facility be given to the child during the study?”
It was observed that only mothers most often did not decide by themselves whether or not to participate in the study and came back after a day or so to give their final response. When both the parents were present, they often took the decision on the spot. In one case, the parents consulted their family physician before deciding to participate in the study.
Of the four cases where only the father had consented, three fathers did not ask any question. One of these was illiterate (consent was obtained in the presence of an impartial witness), one had studied up to middle school, and one was a postgraduate. Three fathers belonged to a rural area, and one to an urban area. The one who had asked questions had studied up to class 10 and belonged to a rural area. His questions were about whether his child would be eligible to continue in the study if they had to leave the city during the study period, whether he could make phone calls in case of any problems, whether the researchers would provide advice about medicines over phone, and so on.
Table 2 presents the background characteristics of the participants, based upon whether they had asked questions during the AV consent process. About 45/100 participants consented to participate in the trial but did not ask any questions. Most of these were both parents present together (30; 66.7%), followed by only mothers (12; 26.7%), and only fathers (3; 6.7%); belonged to rural areas (35; 77.8%) versus urban areas (10; 22.2%); and most had studied up to matriculation (15; 33.3%), followed by graduation and above (9; 22.2%) versus up to primary level (5, 11.1%), middle level (7, 15.6%), illiterate (7; 15.6%) or just literate (1, 2.2%). However, all the illiterate persons (n = 7) who gave consent (in the presence of an impartial witness) did not ask any questions. Among those who gave consent to participate in the study, it was seen that those who were educated up to middle school and matriculation were more likely to ask questions of the researchers compared to those who were educated less than that or more. This association was statistically significant (p = 0.027).
| Table 2: Background characteristics of the participants based upon whether they had asked questions during the audiovisual (AV) consent process | |||||
| Characteristics | Questions asked | Total N = 100 (%) | P value* | ||
| Yes N = 55 (%) |
No N = 45 (%) |
||||
| Persons present at the time of AV consent process | 0.37 | ||||
| Mother and father | 35 (63.6) | 30 (66.7) | 65 (65) | ||
| Mother | 19 (34.6) | 12 (26.7) | 31 (31) | ||
| Father | 1 (1.8) | 3(6.6) | 4 (4) | ||
| Total | 55 (100) | 45 (100) | 100 (100) | ||
| Education of the person who gave consent | 0.02 | ||||
| Illiterate | 0 | 7 (15.6) | 7 (7) | ||
| Just literate | 1 (1.8) | 1 (2.2) | 2 (2) | ||
| Primary school | 2 (3.7) | 5 (11.1) | 7 (7) | ||
| Middle school | 12 (21.8) | 7 (15.6) | 19 (19) | ||
| Matriculation | 29 (52.7) | 15 (33.3) | 44 (44) | ||
| Graduation and above | 11 (20) | 10 (22.2) | 21 (21) | ||
| Total | 55 (100) | 45 (100) | 100 (100) | ||
| Place of residence | 0.707 | ||||
| Urban | 14 (25.5) | 10 (22.2) | 24 (24) | ||
| Rural | 41 (74.5) | 35 (77.8) | 76 (76) | ||
| Total | 55 (100) | 45 (100) | 100 (100) | ||
| *P ≤ 0.05 considered significant | |||||
Table 3 describes some of the queries raised by the subjects’ parents and the pointwise replies given by the authors during the AV informed consent process. All the participants were informed about study procedures of the trial, including the number of visits required, when follow up was required, any related adverse events, and so on, and given the free choice to agree/disagree to participate in the study.
| Table 3: Participant queries and investigator responses during the audiovisual (AV) informed consent process | ||
| Content of the AV consent module | Participant query | Investigator response |
| Study procedure | “Kya hum bahar se bhi yeh tika laga sakte hain?” (Can we obtain this vaccination outside?) | “This particular vaccine is not available in the market. However, there are two other varieties of rotavirus vaccines available in the market manufactured by different companies. But it is not available in government facilities; you have to purchase it from outside.” |
| Length of participation | [As the length of participation was 12–15 weeks long, many participants had some other plans during this period.]
“Hume to gaon jaana hai. Kya hum tike gaon mein laga sakte hain?” (We have to go to our native place. Can we do the vaccination there?) |
“You can go to your village for a week or so, but you have to inform us well before you plan for the trip. However, in case you plan a long trip of 1 month or more, we are afraid we cannot enrol your child in this study. If you are still interested in participating in this study, we suggest you consult with your family members regarding your travel plans in the next 3 months and let us know.” |
| Participant study procedures | “Diary card bharna kya zaruri hai? Kaise bharna hai?” (Is it necessary to fill the diary card? How does one fill it?) | “The diary card helps us to keep a record of the daily health events after vaccination so that any adverse reaction can be picked up early for immediate action. The diary card is very easy to fill (diary card shown to them) with simple questions about the health of your child in the local language. You just have to circle the correct response (demonstration done). In case of any difficulty in filling the card, please feel free to contact us.” |
| Risks and side effects of participation |
[Parents were found to be anxious over the side effects of the vaccine.]
|
“Vaccines are not without any side effects. There are some minor and some major side effects. However the risk of major side effects such as intussusception is very rare. We have to weigh the benefits and risks of administering a vaccine. Moreover, rotavirus vaccines available in the market also have similar risk of side effects. In case of any difficulty, please feel free to contact our project staff on the numbers mentioned in the form at any time of the day. Come immediately to this facility and consult the specialist at room no. 3. In this project, we also have paediatricians from premier tertiary-care institutes as co-investigators. In case of a serious event, we will facilitate your visit to a paediatrician in either of the facilities. We have a referral mechanism in place for any untoward event.” |
| “Agar raat ko bachhe ko koi dikkat ho to kya kare?” (If the child has any difficulties at night, what should we do?) | “In case of inconsolable crying, blood in stools, fever or vomiting, immediately contact our project staff.” | |
| “Humein intussusception ke bare mein kaise pata chale” (How will we know if intussusception has occurred?) “Kitne bachhon ko intussusception hota hai?” (How many children suffer from intussusception?) |
“There is a small risk of intussusception, around 1 in 2000 infants. However, this risk is also present in other licensed rotavirus vaccines available in the market.” |
|
| “Abhi tak jitney bachhon ko apne yeh vaccine pilayi hai, kisi ko aisi dikkat aayi hai?” (Among the children whom you have vaccinated until now, has anyone suffered from this kind of problem?) |
“Until now, no reports of any serious adverse event related to the vaccine, such as intussusception, has come to notice in this project.” |
|
| “Hum to bachhe ko saare tike lagwana chahte hain, lekin agar koi problem hui toh?” (We want to vaccinate our child completely, but in case some difficulties arise, then?) | “In case of any difficulties, don’t hesitate to contact us. We will help you.” | |
| Study visits and vaccination schedule | “Agar hum tike lagana bhul jaye to…?” (If we forget to vaccinate the child, then…?) |
“Don’t worry. We will remind you about the vaccination date of your baby in advance and also on the day of vaccination through phone calls.” |
| “Kya aap saare injection yahan lagaoge?” (Will you give all the vaccinations here?) | “Yes, you will get a full vaccination schedule here for the first three months. After that, we will link you to the nearby government dispensary, where you will get all the subsequent vaccinations.” | |
| “Kya hum BCG vaccination laga sakte hain?” (Can we administer BCG vaccination?) | “Yes, you can administer BCG to the child. There is no problem.” | |
| “Agar aap yahan tike lagaoge toh humein kahin aur lagane ke liye jaana padega kya?” (If you vaccinate my child here, do I need to take him/her elsewhere for other vaccinations?) |
“You will get the full vaccination schedule here for the first three months, following which we will link you to the nearby government dispensary where you will get all the subsequent vaccinations.” | |
| Responsibilities of the participant and termination of participation | “Yadi dispensary mein holiday ho to hum bache ko kahan dikhayen? (If there is a holiday in the dispensary, where should we report?) |
“If the dispensary is closed, please call us. We will facilitate your visit to another nearby hospital that has a 24-hour emergency service.” |
| “Yadi haemin dus din ke liye bahar jana hai to hum ja sakte hain? (If we want to leave town for 10 days, are we allowed to go?) | “Yes, you are free to go anywhere, but you will need to return before the next scheduled visit of your child.” | |
| Other queries | “Kya hum private hospital main dikha sakte hain?” (Can we consult a private hospital?) | “Yes, you can consult any doctor whose qualification is at least MBBS.” |
| “Kya bahar ki dawai ke paise milenge?” (Will you reimburse the money spent on medicines bought outside?) | “Yes, we will reimburse you the money you spend on medicines purchased from outside, but we will need a bill of sale for our records.” | |
Discussion
Although almost a third of enlisted participants refused to participate in the trial before AV consent was brought up, the participation rate in the trial among those who had consented to AV recording was quite high. Below, we discuss the strengths of this process, as observed in the study, which might explain this phenomenon.
Despite fears and apprehensions about the AV consenting process, all the participants in the vaccine trial who expressed initial willingness to be a part of the study agreed to provide AV informed consent without any refusals although they belonged to different cultural groups, genders, socioeconomic status, education levels, and occupations. Explaining the process and purpose of AV consent and addressing the queries seemed to instil confidence among participants in the conduct of the trial.
The AV consent process takes a considerable amount of time, but it is a one-time activity and deserves the time it warrants. It has been reported in other studies that AV recording of clinical trial consent increases the transparency of the informed consent process, which is similar to the findings of this study. All the participants were told about each and every aspect of the trial and given a free chance to raise queries related to the trial and to agree/disagree to give consent to participate (1). This reassures the regulatory authority about the practice of clinical trial standards and ethics and re-establishes society’s faith in clinical research.
AV recording does not only protect the rights, safety, and well-being of the subjects enrolled in the trial but actually plays a key role in safeguarding the interests of all stakeholders in the trial (1). In case of any dispute/litigation, the investigator will be able to demonstrate hard evidence that all relevant information was provided to the potential participant before s/he confirmed understanding and voluntarily agreed to take part in the clinical study. Introduction of the AV recording could also improve how the informed consent process is conducted because the process is recorded. A systematic review by Synnot et al. (10) reported that AV consent improves participant satisfaction with the consent information provided. AV consent also implies that conduct is given more importance over mere documentation of the whole process. This ensures that incidents like the one in which irregularities in the conduct of study and informed consent in a Human Papilloma Virus vaccine trial were reported do not happen again (11, 12).
Unsurprisingly, most of the queries reported in this trial during the AV consent process were related to apprehensions about risks to the subject as a result of the vaccine. The minor side effects were already known to most parents, but the major risk—intussusception due to the rotavirus vaccine—was a cause of concern for most parents. However, the authors felt that discussion with the anxious parents allayed most of their fears. It also helped build a healthy rapport between the parents and the investigators.
Parents were apprehensive about where to consult in case of any emergencies. The trial had clearly written standard operating procedures for referral and management in case of any emergency in collaboration with the departments of paediatrics and radiodiagnosis at nearby tertiary-care health facilities, which were around 4–6 kilometres from the study area. This was told to each participant during AV consenting. The mobile number of the project field staff was shared with the participants. The parents were also given the option of consulting any private physician if needed. In the event of any private consultation, they were reimbursed any expenditure incurred.
The decision to participate / not participate in the study, in most of the cases, were found to be taken jointly by both mother and father; however, fathers were found to give final approval. The women who were more educated were able to ask more questions regarding the vaccine trial and were able to take independent decisions irrespective of their residential background (rural or urban).
Another fact that might have led to better participation rates is that the study investigators belong to a reputed medical institute in the region and have been providing medical care in the region for many years as part of a community outreach programme. They have also been involved in door-to-door primary health service delivery through a team of field workers. This would likely have facilitated initial trust building.
There were quite a few questions about the existing vaccination schedule of the infant and any change in it as a result of this vaccine. As the trial was being conducted adjacent to the Maternal and Child Health Clinic, within the study hospital, the infants were linked to the nearby immunisation centre for subsequent vaccinations, which also reassured the parents. The project staff ensured complete vaccination of the child as per schedule and facilitated the process in every visit, which also helped in building personal rapport.
The limitations of the study include that it reviews the AV consent process in a single institution among parents of healthy infants; therefore, the results of this study may not be generalisable. These results might however be applicable to other sites, as the settings for AV consenting tend to be very similar at all the sites, as per the Central Drugs Standard Control Organization guidelines (13).
This study captured the AV consent process of 100 participants qualitatively. For qualitative analysis, 25–30 observations can often be sufficient to provide a picture, depending upon the stage when the data saturation is achieved. Hence, we feel that obtaining observations from 100 participants in this study are adequate to provide conclusive evidence. Since we retrospectively reviewed the AV recordings and did not prospectively interview the participants after the AV consent process to explore their understanding regarding the clauses mentioned in the consent form, we cannot comment on these. Due to this limitation in the methodology, the satisfaction level of the participants after getting replies from the authors could also not be observed. The Shilling et al (14) qualitative study explored the perceptions of the practitioners and parents regarding participation in clinical trials. The authors observed that, contrary to the beliefs of the practitioners, parents were more positive, comfortable, and viewed participation in the clinical trials as an exciting opportunity.
The Shetty et al (15) study highlighted many challenges in the AV consent process including non-availability of infrastructure, image and sound quality, duration of recording, testing the understanding of participants, training of personnel, and storage archival and retrieval of video recordings. However, no such problems occurred at this study site.
Conclusions
The participation rate in this vaccine trial, among those who underwent the AV consent process, was very high due to the descriptive and rigorous process followed. The findings of this study will assist researchers involved in conducting vaccine/clinical trials to understand the importance of the AV consent process and build their capacity to narrate all the study procedures in detail, including the likely side effects of the investigational product. It will also assist them in dealing with participant queries with increased confidence. It is recommended that, in addition to implementing the guidelines of the Central Drugs Standard Control Organization, Government of India, regarding the AV consent process, the regulatory agencies further standardise this process by (i) formulating a list of frequently asked questions with answers supplied by individual study investigators and (ii) specifying the technical specifications of devices to be used for AV recording.
Competing interests: None declared
Funding support: The vaccine trial was funded by Shantha Biotech Limited (a part of Sanofi company).
Acknowledgements
We would like to thank Mrs. Seema Sharma, project nurse, for transcribing and translating the AV recordings of the subjects.
References
- Kulkarni NG, Dalal JJ, Kulkarni TN. Audio-video recording of informed consent process: boon or bane. Perspect Clin Res. 2014 Jan;5(1):6-10.
- Central Drugs Standard Control Organization. Drugs and Cosmetics Rules 1945, Schedule Y: Requirements and guidelines for permission to import and/or manufacture of new drugs for sale or to undertake clinical trials. New Delhi: Ministry of Health and Family Welfare, Government of India; n.d. Available from: http://cdsco.nic.in/html/D&C_Rules_Schedule_Y.pdf
- Kumar NK. Informed consent: past and present. Perspect Clin Res. 2013 Jan; 4(1): 21-5.
- Central Drugs Standard Control Organization. Drugs and Cosmetics Rules 1945, Amendment Notification. New Delhi: Ministry of Health and Welfare, Government of India; 2013 Jun 7. The Gazette of India; Extraordinary Part II, Section 3, Sub-section (i), G.S.R 364 (E). Available from: http://cdsco.nic.in/writereaddata/GSR%20364Ejune13.pdf
- Bhatt A. India’s next challenge: rebooting recruitment. Perspect Clin Res. 2014;5(3):93-4.
- Gitanjali B, Raveendran R, Pandian DG, Sujindra S. Recruitment of subjects for clinical trials after informed consent: does gender and educational status make a difference? J Postgrad Med. 2003 Apr-Jun;49(2):109-13.
- Chauhan RC, Purty AJ, Singh N. Consent for audio-video recording of informed consent process in rural South India. Perspect Clin Res. 2015 Jul-Sep;6(3):159-62.
- Devakumar D, Brotherton H, Halbert J, Clarke A, Prost A, Hall J. Taking ethical photos of children for medical and research purposes in low-resource settings: an exploratory qualitative study. BMC Med Ethics. 2013 Jul 9;14:27.
- Saluja T, Palkar S, Misra P, Gupta M, Venugopal P, Sood AK, Dhati RM, Shetty A, Dhaded SM, Agarkhedkar S, Choudhury A, Kumar R, Balasubramanian S, Babji S, Adhikary L, Dupuy M, Chadha SM, Desai F, Kukian D, Patnaik BN, Dhingra MS. Live attenuated tetravalent (G1-G4) bovine-human reassortant rotavirus vaccine (BRV-TV): randomized, controlled phase III study in Indian infants. Vaccine. 2017 Jun 16;35(28):3575-81.
- Synnot A, Ryan R, Prictor M, Fetherstonhaugh D, Parker B. Audio-visual presentation of information for informed consent for participation in clinical trials. Cochrane Database Syst Rev. 2014 May 9;(5):CD003717.
- Mudur G. Human papillomavirus vaccine project stirs controversy in India. BMJ. 2010 Mar 29;340:c1775.
- Sarojini N, Deepa V. Trials and tribulations: an expose of the HPV vaccine trials by the 72nd Parliamentary Standing Committee Report. Indian J Med Ethics. 2013 Oct-Dec;10(4):220-2.
- Central Drugs Standard Control Organization. Draft guidelines on audio-visual recording of informed consent process in clinical trial. New Delhi: Ministry of Health and Family Welfare, Government of India; 2014 Jan 9. Available from: http://www.cdsco.nic.in/writereaddata/Guidance_for_AV%20Recording_09.January.14.pdf
- Shilling V, Williamson PR, Hickey H, Sowden E, Beresford MW, Smyth RL, Young B. Communication about children’s clinical trials as observed and experienced: qualitative study of parents and practitioners. PLoS One. 2011;6(7):e21604.
- Shetty PA, Maurya MR, Figer BH, Thatte UM, Gogtay NJ. Audiovisual recording of the consenting process in clinical research: experiences from a tertiary referral center. Perspect Clin Res. 2018 Jan-Mar;9(1):44-7.
