RESEARCH ARTICLE
In Clinical Trials Registry-India, the classification of sponsors needs to be standardised
Jaishree Mendiratta, Mounika Pillamarapu, Gayatri Saberwal
Published online first on November 18, 2023. DOI:10.20529/IJME.2023.071Abstract
Background: In recent years, there has been a big push to register trials, but there are a number of problems with the data in public clinical trial registries. Here, we describe a cross-sectional study of the classification of the primary sponsors of all Phase 2, Phase 2/3, and Phase 3 interventional trials registered with the Clinical Trials Registry-India (CTRI) between May 15, 2016 and May 14, 2021.
Methods: Data was scraped from the records of CTRI, various filters were applied, and the trials of interest identified.
Results: Of 5,453 trials, 105 did not identify a sponsor and 1,080 were sponsored by individuals. Of the remaining 4,268 trials, 427 had unique sponsors, and 3,841 had a total of 350 non-unique sponsors. Of the 350 sponsors, 202 were classified in a single category, and 147 were classified in two or more categories. Overall, of the 3,841 trials, sponsors in 3,537 (92.1%) were classified in one or more of nine well-defined categories, and 304 (7.9%) were classified as various versions of “Other”. Three major problems with the sponsor data were identified: each trial does not necessarily list a sponsor, a given sponsor may be categorised in multiple ways, and there has been an excessive use of the “Other” category. Addressing these problems will enable automated analyses of the database, and improve the transparency of the data.
Conclusion: Our study generates evidence highlighting the need to improve the trial registration system in India, and perhaps elsewhere.
Keywords: CTRI, clinical research, data integrity, trial registry‐metaresearch
Background
Clinical Trials Registry-India (CTRI) was established in July 2007. Since June 2009 it has been mandatory to register trials running in India with CTRI [1], and prospective registration has been mandatory since April 01, 2018 [2]. The number of registered trials has rapidly increased in recent years [3], and as of November 03, 2023, the registry held records of 59,532 trials [4].
The World Health Organization (WHO) recognises: (i) 17 registries, including CTRI, as primary registries [5], and (ii) three registries, including ClinicalTrials.gov, the registry of the United States, as a “data provider” [6]. As of November 03, 2023, 18 of these 20 registries collectively held records of 8,19,891 trials. CTRI was the fourth in terms of the number of records of the 18 registries. As one of the bigger public trial registries, CTRI is an important one for analyses of trial data.
Over the years, researchers have, broadly, performed two kinds of studies with the data in CTRI.
1. They have summarised data hosted by CTRI, or posed particular questions about the registered trials. These studies looked at questions such as (i) the year-wise distribution of registrations [7, 8]; (ii) the nature of the trials [8, 9, 10]; (iii) the phase-wise distribution of trials [8, 9, 10, 11]; (iv) the regions of the country in which studies were carried out [7, 8, 9, 10]; (v) the conditions that were studied [7, 9, 10]; (vi) the categories of sponsors [9, 10, 12, 13]; (vii) whether surrogate endpoints were assessed more frequently in company-sponsored trials [12]; (viii) whether methods were better described in CTRI records than in the publications reporting the trials [14]; (ix) whether patients with rare diseases who were based in India were included in multinational trials [15]; and (x) the ethnic representation in trials run in India [16].
2. They have identified several problems with the CTRI records. These include data that are missing, incomplete or non-standard; internal inconsistencies in the information in various fields; the name of a given person represented in multiple ways; and missing or incomplete details of ethics committees [3]. In addition, some fields may not have been updated, and there are insufficient links to further information [17]. Studies also found trials that had not been registered [17, 18], and evidence of a faulty search function that could lead to an incomplete set of trials being identified during a given search [3]. It is important to document such issues so that the trial records are interpreted with suitable caution and give increased confidence in a particular analysis.
The CTRI website hosts a document that explains what each field in a CTRI record means [19]. Part of the description of the sponsor reads, “…the individual, organization, group or other legal person taking responsibility for securing the arrangements to initiate and/or manage a study… The Primary Sponsor is responsible for ensuring that the trial is properly registered. The Primary Sponsor may or may not be the main source of funding…”.
For anyone registering a trial with CTRI [4], the registry provides the following 10 sponsor classification options: (i) Contract research organization, (ii) Government funding agency, (iii) Government medical college, (iv) Pharmaceutical industry-Global, (v) Pharmaceutical industry-Indian, (vi) Private hospital/clinic, (vii) Private medical college, (viii) Research institution, (ix) Research institution and hospital, and (x) Other. There is a free-text field to provide more details in the “Other” category.
Our earlier work indicated that a given Primary Sponsor (hereafter sponsor) was sometimes classified in multiple ways [3], and we wished to explore this further. Here, we have investigated the variation in the classification of a given sponsor of trials registered with CTRI.
Further, we wished to compare the number of sponsor categories used by CTRI with the categories used by other prominent registries. For this, we examined three of the best rated [20] WHO-recognised registries: the United States’ ClinicalTrials.gov, the Australia New Zealand Clinical Trial Registry (ANZCTR), and South Korea’s Clinical Research Information Service (CRIS).
Methods
Sampling strategy and data extractionThe website http://ctri.nic.in/Clinicaltrials/advancesearchmain.php hosts the CTRI records [Links to sample CTRI records are provided in S1 File (available online only)]. As of May 15, 2021, the database held 33,620 records. Using a script in R programming language that was developed in-house [S2 File (available online only)], data were extracted from the CTRI records, to identify interventional trials registered with CTRI between May 15, 2016 and May 14, 2021, that were in Phase 2, Phase 2/3 or Phase 3.
Data pre-processing and cleaningThe data were cleaned, processed, and stored in a SQLite database [S3 File, https://osf.io/p7432 (available online only)]. The database schema is provided in S4 File (available online only). Other details of the methodology are provided in S5 File (available online only).
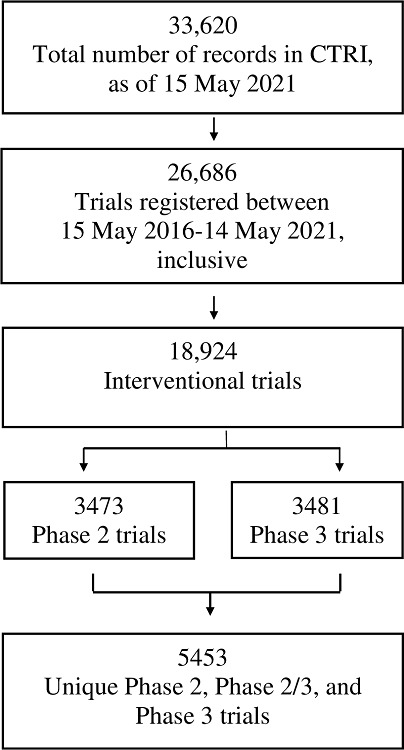
There were 26,686 trials registered over the 5-year period of our study. We followed the steps outlined in Figure 1, and in S6 File (available online only) and S7 File (available online only), leaving 5,453 unique trials for analysis.
Figure 1. Steps taken to identify the set of 5,453 unique, interventional, Phase 2, Phase 2/3 and Phase 3 trials registered with CTRI between 15 May 2016 and 14 May 2021.
Descriptive analysis
The name and classification of the sponsor of each of the 5,453 trials is listed in S7 File (available online only). Next, for convenience in handling the data, we created 26 sheets, with each sheet listing the sponsors’ names that started with one letter of the alphabet. On a given sheet, all the trials hosted by sponsors whose names started with a particular letter were listed. All the trials by a single sponsor were grouped together, including if the sponsor was represented by name variants. Some sponsors had only one trial to their name; all such “single” sponsor cases were grouped together, on the same sheet. Additionally, one sheet was created for cases where no sponsor was listed, and one sheet for those that listed individuals as the sponsors. These 28 sheets are available in S8 File (available online only).
By way of a limited comparison of how sponsors are classified in various registries, we looked at the classification of sponsors in ClinicalTrials.gov [21], ANZCTR [22] and CRIS [23], and downloaded a sample of trials from each registry to examine any variations in the categories.
After the creation of the local SQLite database, two authors independently performed each step of the methodology.
Results
The 5,453 trials could be classified in four groups (Figure 2): In 105(2%) trials, the sponsor name was listed as NA/nil/not applicable/no/none etc. In 1,080(20%) trials, an individual was named as the sponsor. In 427(8%) trials, a unique sponsor (that had sponsored just one trial), that was not an individual, was listed. In 3,841(70%) trials, a total of 350 non-unique sponsors (that had sponsored two or more trials) that were not individuals, were listed.
Figure 2. Steps taken to identify the 147 sponsors that sponsored two or more trials, and that were classified in multiple ways.
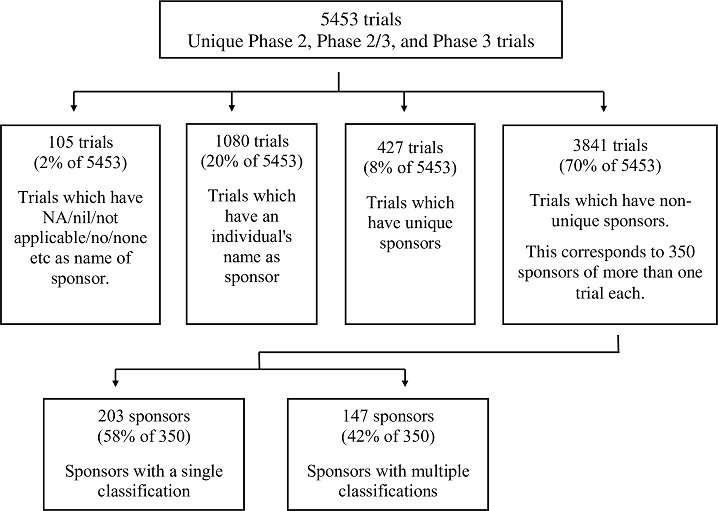
We examined the 350 sponsors of multiple trials each. Of these, only 203(58%) were classified in the same category across trials. One organisation, the Government Siddha Medical College, Chennai, stood out because for a large number of trials (ie 89), it had a single classification, ie Government medical college. The remaining 147(42%) sponsors were classified in two or more ways in the trial registry.
For each of these 147 sponsors, we calculated the percentage of trials that were in the single largest classification category. For example, the All India Institute of Medical Sciences (AIIMS), New Delhi, sponsored 164 trials. Across these 164 trials, it was classified differently in different trials, as follows: Research institution and hospital (107 trials, 65%), Government medical college (42, 26%), Research institution (12, 7%), Government funding agency (2, 1%), and Other [Research Centre in Government medical institution] (1). “Research institution and hospital” was the single largest category, accounting for 65% of the studies sponsored by AIIMS, New Delhi. We determined the distribution of the single largest categories for all 147 sponsors, and binned them in Table 1 [further details are given in S9 File (available online only)]. Since the largest percentage of trials in the AIIMS case described above was 65%, it was classified in row 6 of Table 1. The lowest percentage for the single largest category of sponsor category for one organisation was 25%, and the highest was 98%.
|
Table 1. For each of the 147 sponsors with more than one classification, the distribution of the largest percentage of trials with a particular classification. |
||||
|
S. No. |
Percentage range of the single largest classification category of a given sponsor |
Number of sponsors |
Percentage of 147 trials |
Cumulative percentage of 147 trials |
|
1 |
11–20% |
0 |
0.0 |
0 |
|
2 |
21–30% |
2 |
1.4 |
1.4 |
|
3 |
31–40% |
4 |
2.7 |
4.1 |
|
4 |
41–50% |
43 |
29.3 |
33.3 |
|
5 |
51–60% |
11 |
7.5 |
40.8 |
|
6 |
61–70% |
31 |
21.1 |
61.9 |
|
7 |
71–80% |
25 |
17.0 |
78.9 |
|
8 |
81–90% |
17 |
11.6 |
90.5 |
|
9 |
91–99% |
14 |
9.5 |
100.0 |
|
TOTAL |
147 |
100 |
||
We then identified the organisations that were classified into five or more categories, and made two observations:
1. The organisations with the largest number of categories were the National Institute of Ayurveda (15 categories, with 80% being various versions of “Other” such as Other [Ayurveda University], Other [Autonomous Institution under Ministry of AYUSH], and Other [Ayurveda College]); the Tata Memorial Centre/Tata Memorial Hospital (10, with 70% Other); and the Sri Dharmasthala Manjunatheshwara College of Ayurveda and Hospital/SDM Ayurveda Hospital (9, 56% Other).
2. The organisations with the smallest fraction of “Other” were Post Graduate Institute of Medical Education and Research, Chandigarh, and the All India Institute of Medical Sciences, New Delhi (each with 5 categories, with 20% being various version of Other), and the Parul Institute of Ayurved/Parul University (7, with 29% Other) [S9 File (available online only)].
As mentioned earlier, a total of 3,841 trials were sponsored by the 350 sponsors of multiple trials each. Of these, 3,537(92.1%) trials’ sponsors were classified in the nine well-defined categories, and 304 (7.9%) were classified in various versions of “Other” [S9 File (available online only)].
We noted that there were several problems with sponsors’ classifications. Specific examples of these problems are listed in Table 2, with URLs for these examples provided in S10 File (available online only).
| Table 2. Examples of trials that illustrate various problems with the sponsor classification or name | ||||
|
S No |
Nature of problem |
Sponsor name |
CTRI Number |
Sponsor classification |
| Issues with sponsor classification | ||||
|
1 |
Non-sensical sponsor category |
University College London |
CTRI/2018/10/015931 |
[Public Research] |
|
2 |
Clearly incorrect sponsor category |
Ayothidoss pandithar hospital |
CTRI/2019/05/019123 |
Other [research student] |
|
World Health Organization |
CTRI/2016/06/006996 |
Government funding agency |
||
|
3 |
Contradictory sponsor categories |
Mahesh Bhattacharyya Homoeopathic Medical College Hospital |
CTRI/2018/08/015329 CTRI/2018/08/015313 |
Government medical college Private medical college |
|
4 |
Apparently incorrect sponsor category |
The Wellcome Trust DBT India Alliance |
CTRI/2017/08/009296 |
Government funding agency |
|
5 |
Many levels within the organization that have been listed as the sponsor across trials. |
Manipal College of Nursing Manipal Manipal University Manipal Academy of Higher Education Center for Sport Science Medicine and Research Innovation Center |
CTRI/2016/11/007449 CTRI/2017/09/009854 CTRI/2020/02/023371 CTRI/2017/06/008875 CTRI/2019/01/016950 |
Research institution Private medical college Research institution and hospital Other [Research institution and hospital] Research institution and hospital |
|
6 |
The ‘other’ category is prone to proliferation and verbose descriptions. |
Atlantic Coast Brands |
CTRI/2018/03/012868 CTRI/2018/05/013975 |
Other [Marketer & manufacturer of phytonutrients, ayurvedic, herbal extracts, probiotics etc.] Other [International beauty company that builds iconic beauty brands.] |
|
7 |
A proliferation of similar sponsor categories |
Arjuna Natural Limited |
CTRI/2018/07/014792 CTRI/2018/04/013154 |
Other [Neutraceutical Industry] Other [Nutraceutical Industry] |
|
Issues with sponsor name |
||||
|
8 |
No sponsor name listed |
NA |
CTRI/2018/05/014198 |
Other [NA] |
|
9 |
Sponsor name’ field listed positions such as Dean/ Director/Incharge/Investigator/Principal/Superintendent |
The Director |
CTRI/2017/09/009858 |
Government funding agency |
|
10 |
The agency that funded the organization, or the concerned project listed as the sponsor. |
World diabetes foundation |
CTRI/2017/02/007945 |
Research institution and hospital |
|
11 |
The use of ambiguous sponsor names or acronyms |
All India Institute of Medical Sciences1 All India Institute of Medical Sciences2 All India Institute of Medical Sciences3 |
CTRI/2019/01/017003 CTRI/2017/11/010478 CTRI/2017/04/008385 |
Government medical college Research institution and hospital Government medical college |
|
1 In Jodhpur, Rajasthan 2 In Bhubaneswar, Orissa 3 In New Delhi |
||||
Of the 5,453 trials, 105 did not list a sponsor name at all. Instead, the Primary Sponsor: Name field listed designations such as Dean, Director, Incharge, Investigator, Principal, or Superintendent. Multiple organisations used a common name or acronym.
We compared CTRI with three other prominent registries. Although ClinicalTrials.gov did not provide a choice of category of Sponsor in its search function, it did provide a choice of four categories of Funder Type, ie. NIH, Other US Federal agency, Industry, and All others (individuals, universities, organisations). ANZCTR listed eight categories for the Primary sponsor type under its Advanced search option, ie. (i) Government body, (ii) Hospital, (iii) University, (iv) Commercial sector/Industry, (v) Charities/Societies/Foundations, (vi) Other collaborative groups, (vii) Individual, and (viii) Other. CRIS had the following categories: (i) Pharmaceutical company, (ii) Medical institute, (iii) Research institute, (iv) University, (v) Government and (vi) Others. By downloading a sample of trials from each of these three registries, we determined that none of them had a free-text field for sponsor classification, either as a standalone comment, or linked to any of the other categories.
We compared CTRI with three other prominent registries. Although ClinicalTrials.gov did not provide a choice of category of Sponsor in its search function, it did provide a choice of four categories of Funder Type, ie. NIH, Other US Federal agency, Industry, and All others (individuals, universities, organisations). ANZCTR listed eight categories for the Primary sponsor type under its Advanced search option, ie. (i) Government body, (ii) Hospital, (iii) University, (iv) Commercial sector/Industry, (v) Charities/Societies/Foundations, (vi) Other collaborative groups, (vii) Individual, and (viii) Other. CRIS had the following categories: (i) Pharmaceutical company, (ii) Medical institute, (iii) Research institute, (iv) University, (v) Government and (vi) Others. By downloading a sample of trials from each of these three registries, we determined that none of them had a free-text field for sponsor classification, either as a standalone comment, or linked to any of the other categories.
In summary, of 5,453 interventional Phase 2, Phase 2/3 and Phase 3 trials, 105 did not identify a sponsor, and 1,080 were sponsored by individuals. Of the rest, 427 trials had unique sponsors, and 3,841 had non-unique ones. A total of 350 sponsors sponsored the latter set of 3,841 trials. Of these, 202(58%) sponsors were classified in a single category, and 147(42%) were classified in two or more categories. The maximum number of classifications for one sponsor was 15. Overall, of 3,841, 3,537(92.1%) trials’ sponsors were classified in the nine well-defined categories and 304(7.9%) were classified as various versions of “Other”.
Discussion
The present study documents problems with the data entered in the Primary sponsor field of several trials registered with CTRI. Based on these findings, we have made recommendations for consideration by the managers of CTRI, as to how the data in this field could be improved.
It is known that registries have problems with their data, underlining the need to register trials, and for the registry data to be accurate [24, 25, 26]. It is also known that the names of sponsors in trial registries have variations. For example, there have been reports of 123 variants of the name GlaxoSmithKline [27] and 51 variants of the University of Leuven [28] in registries. However, we were unaware of any reports of variations in sponsor classification in other, non-Indian, registries, or any systematic study of such variations. Here, we investigated variations in the classification of a given sponsor in the CTRI records.
As mentioned, many trials in CTRI did not list a sponsor name. In examining some of these records, it became clear that the word “sponsor” was being interpreted as a funder, often as an external funding agency, but “sponsor” and funder have different definitions. Since at least 2013, the managers of CTRI have been aware that registrants do not always understand the meaning of particular terms used in the registry [29]. The managers of ClinicalTrials.gov have also suspected that sponsors or Principal Investigators sometimes do not correctly interpret the requirements of a field in their registry [30]. Going by the instructions in the E-tutorial on the CTRI website, it does not appear to be mandatory for each registrant to undergo suitable training before registering a trial. Such a programme is recommended.
The study found that 147 (close to 50%) of the 350 sponsors of multiple trials were classified in multiple ways. As is clear from Table 1, the single largest category for a particular sponsor was as low as 25% of the occurrences of that sponsor. Additionally, one organisation was classified in 15 ways, including Government medical college, Research institution and hospital, Other [Ayurveda University], Other [Autonomous Institution under Ministry of AYUSH], Other [Ayurveda College], Other [Ayurveda Medical college], Other [Government Medical college], Other [National Institute of Ayurveda] and so on. . Yet another issue relates to the listing of different levels of units within an organisation, or of the agency that funds the organisation or the project, for instance, which creates heterogeneity that serves no purpose. Examples of this are available in S8 File (available online only), and include (a) the National Institute of Siddha and its constituent hospital, the Ayothidoss Pandithar Hospital and (b) AIIMS, Delhi and its various departments such as the Centre for Dental Education and Research, and the Dr Rajendra Prasad Centre for Ophthalmic Sciences.
Although we did not encounter this in our sample of trials, another potential issue relates to ambiguity in sponsor acronyms. There are several All India Institutes of Medical Sciences in the country, all of which are abbreviated as AIIMS; at least five organisations, the Kalinga, Karnataka, Kerala, and Krishna Institutes of Medical Sciences and the Kempegowda Institute of Medical Sciences Hospital, share the acronym KIMS [31]. This could complicate an analysis, since it might then require an examination of the address field to identify the sponsor.
The “Other” category of sponsor classification was used in almost 8% of the trials by sponsors with more than one trial each. Moreover, due to the free-text field, there were cases of verbose descriptions, which led to a proliferation of “Other” categories. Even where the classifications of various versions of “Other” were similar, they would not have been counted as the same in any automated data analysis. Ways to minimise the use of “Other” need to be explored.
Overall, we noted several problems with sponsors’ classifications: (i) The classification may have been nonsensical, contradictory, clearly incorrect, or apparently incorrect; (ii) Across the multiple trials with a common sponsor, different levels of units within the organisation may have been listed as the sponsor; and (iii) There may have been a proliferation of descriptions in the “Other” category. Each of these issues may contribute to poor data quality for particular trials, and also jeopardise any analysis across trials. In general, it is important that a single name and a single classification (in a standard format) be entered in the pertinent fields, as has been done in ClinicalTrials.gov [32]. This will enable an unambiguous analysis of how many trials a given organisation has sponsored, and how many organisations are in a given category of sponsors.
In a comparison of CTRI and the three foreign registries, we believe that ClinicalTrials.gov’s four categories of funders are inadequate. Both ANZCTR and CRIS, like CTRI, have more categories to classify their sponsors. Researchers studying sponsors have created nine categories to better understand sponsors [33]. CTRI provided nine specific sponsor categories, compared to ANZCTR’s seven. However, it is clear from some of the examples of sponsors classified as “Other”, as listed above, that the list of nine categories is inadequate. Currently, sponsors such as a private dental college, biotechnology company, central university, charity organisation, or children hospital, for instance, have no option but to classify themselves as “Other”. Expanding the number of categories or grouping some types of organizations into one category would help with such cases.
The fact that the categories “Pharmaceutical industry-Global” and “Pharmaceutical industry-Indian” have been created indicates that there is an interest in which sponsors are non-Indian entities. But in no other category is there a provision to distinguish global and Indian entities. A separate sub-field can be created regarding whether the entity is Indian or foreign. Either the list of categories needs to be expanded or different levels of classification need to be introduced, such as foreign or Indian; corporate, government, or non-profit; and so on. One question that CTRI managers may wish to decide upon is how to classify an Indian company or other organisation that has expanded its activities to other countries. Should it be classified as a multinational company, or perhaps as an Indian multinational company?
There also need to be clear instructions for the basis of choosing a particular category, and these guidelines need to be enforced. CTRI’s IT systems ought to be strengthened to prevent some of these errors. This must be accompanied by extra vigilance on the part of the registry’s staff when they inspect the submitted records.
Other registries have also struggled with quality issues. ClinicalTrials.gov staff have instituted automated systems, and also manual checks of the submitted records [30], and only after a trial passes quality control [34] is it posted on the ClinicalTrials.gov website. It has been estimated that only one-third of trials that are submitted to this registry are good enough to be made public immediately [35]. Additionally, at the sponsors’ end, some institutions have created small teams to help improve the quality of their submitted records, and generally to be in compliance with trial registration requirements [36].
Our findings suggest the following action items. First, an individual, group, or organisation must be responsible for each trial, and therefore each trial must list a sponsor. Second, a given sponsor needs to have a single name and classification, to enable automated analyses of the sponsors of all the trials in CTRI. Third, there has been an unnecessarily large use of the category “Other” to categorise the sponsors. This needs to be cut down by either expanding the number of categories or redesigning the system of sponsor categories, with clearer definitions of each category. Fourth, as recommended for ClinicalTrials.gov, sponsors need to receive more detailed guidance to improve their registration practices [32]. There could be mandatory e-training, at least, before registering a trial. Fifth, there could be a separate sub-field regarding whether the entity is Indian or foreign, with clarity on how an Indian multinational organisation should be classified. Sixth, although significant effort already goes into ensuring that CTRI trial records are of high quality [29], registry staff need to improve the IT system and their inspection of trial records before accepting them. Seventh, as recommended for trial records in the European Union [32], there should be adequate resources for monitoring quality and also for regular audits of CTRI records. Eighth, although we have focused on the primary sponsor, some of these actions should extend to the fields Details of Secondary Sponsor and Source of Monetary or Material Support as well. Finally, some of the problems identified in the CTRI records may also exist in the records of some of the other public registries. Therefore, suitable action needs to be taken by those registries to improve their records as well.
This study has some limitations. First, we only studied the records held by CTRI. Therefore, we are unable to comment on how well sponsors are classified in all the other WHO-recognised registries, or in the documents of trials not registered with CTRI. Second, we only studied a recent five-year period of records. Third, we did not investigate whether, over the five years of this study, there was an improvement or a worsening of the classification of sponsors. And fourth, we only studied Phase 2, Phase 2/3 and Phase 3 interventional trials. It is possible that for other kinds of trials, or those conducted outside this five-year window, other, unidentified, issues related to the sponsors’ names, or classification may exist.
Many individuals and organisations, including the United Nations [37], have spoken about the need for increased transparency as crucial to sound governance. The free public availability of data and the ability to perform automated analyses of these data are important to achieve this. The implementation of the action items listed above would improve the data quality and data transparency in CTRI and other public clinical trial registries. This should better serve the interests the general public, patients, the medical community, policy makers, health researchers, companies, non-profits, and funders of biomedical research.
Ethics committee approval: Not applicable.
Authors’ contributions: Gayatri Saberwal: Conceptualisation, formal analysis, funding acquisition, methodology, validation, visualisation, project administration, writing the original draft and reviewing and editing it; Jaishree Mendiratta: Data curation, investigation, formal analysis, methodology, software, visualisation, reviewing and editing the final draft; Mounika Pillamarapu: Investigation, reviewing and editing the final draft. All authors approved the final draft.
Conflict of interest: In 2021, Gayatri Saberwal was commissioned by public health activist Mr Dinesh Thakur to write a report on the various ways in which the workings of the CTRI could be improved. She submitted a report with 18 points. The issue discussed in this manuscript was one of the 18. She received consulting fees for this work.
Acknowledgments: We are grateful to Indraneel Chakraborty for creating the SQLite database.
Funding: This work was supported by internal funding of the Institute of Bioinformatics and Applied Biotechnology, from the Department of Electronics, Information Technology, BioTechnology and Science & Technology of the Government of Karnataka. The funder had no role in study design; in the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication. There was no commercial funding for the work. None of the authors have received any commercial funding for any other work either.
Statement of similar work: A similar analysis has not been performed before, either by these authors or any other.
References
- Selvarajan S, George M, Kumar SS, Dkhar SA. Clinical trials in India: Where do we stand globally? Perspect Clin Res 2013;4(3): 160–164. https://doi.org/10.4103/2229-3485.115373
- 2. Chakraborty I, Saberwal G. CTRI requirement of prospective trial registration: Not always consistent. Indian J Med Ethics. 2022;VII(4): 312–314. https://doi.org/10.20529/IJME.2022.033
- Pillamarapu M, Mohan A, Saberwal G. An analysis of deficiencies in the data of interventional drug trials registered with Clinical Trials Registry – India. Trials. 2019;20: 535. https://doi.org/10.1186/s13063-019-3592-0
- Clinical Trials Registry – India. ICMR National Institute of Medical Statistics. Cited on 2023 June 30. Available from: http://ctri.nic.in
- World Health Organization. International Clinical Trials Registry Platform. ICRTP Registry Network. Primary registries. Cited on 2023 June 28. Available from: https://www.who.int/
- World Health Organization. International Clinical Trials Registry Platform. ICRTP Registry Network. Data providers. Cited on 2023 June 28. Available from: https://www.who.int/
- Chaturvedi M, Gogtay N, Thatte U. Do clinical trials conducted in India match its healthcare needs? An audit of the Clinical Trials Registry of India. Perspect Clin Res. 2017[Cited on 2023 Nov 03];8(4): 172–175. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5654216/
- Bhide SS, Tadvi F, Maurya M, Bhojne S, Chandrakar P. Assessment of clinical trials registered at clinical trial registry of India over past decade: an audit. Int J Clin Trials. 2016;3(4): 238–243. http://dx.doi.org/10.18203/2349-3259.ijct20163187
- Ravindran D, Nikarge S. Clinical Trials Watch. Indian J Med Ethics. 2010;VII(2): 127–129. https://doi.org/10.20529/IJME.2010.047
- Nikarge S, Pamnani D. Clinical Trials Watch. Indian J Med Ethics. 2009;VI(4): 228–231. https://doi.org/10.20529/IJME.2009.082
- Ravindran D. Clinical Trials Watch. Indian J Med Ethics. 2013;X(1): 73. Doi: https://doi.org/10.20529/IJME.2013.025
- Yadav P, Jaykaran, Chaudhari M, Saxena D, Kantharia ND. Clinical trials registered in clinical trial registry of India: A survey. J Pharmacol Pharmacother. 2011;2(4): 289–292. https://doi.org/10.4103/0976-500X.85953
- Jacob VD, Ravindran D, Ved K. Clinical Trails Watch. Indian J Med Ethics. 2012;IX(1): 73. https://doi.org/10.20529/IJME.2012.024
- Tharyan P, George AT, Kirubakaran R, Barnabas JP. Reporting of methods was better in the Clinical Trials Registry-India than in Indian journal publications. J Clin Epidemiol. 2013;66(1): 10–22. https://doi.org/10.1016/j.jclinepi.2011.11.011
- Chakraborty M, Choudhury MC, Chakraborty I, Saberwal G. Rare disease patients in India are rarely involved in international orphan drug trials. PLOS Glob Public Health. 2022;2(8): e0000890. https://doi.org/10.1371/journal.pgph.0000890
- 16. Mendiratta J, Pillamarapu M, Chakraborty I, Vaswani R, Kapoor M, Vadlamani S, et al. Ethnic representation in interventional clinical trials run in India. The Lancet Reg Health – Southeast Asia. 2023;15: 100230. https://doi.org/10.1016/j.lansea.2023.100230
- George B. CTRI – Clicking to greater transparency and accountability. Perspect Clin Res. 2012;3(4): 122–124. https://doi.org/10.4103/2229-3485.103592
- Kumari S, Mohan A, Saberwal G. Hidden duplicates: 10s or 100s of Indian trials, registered with ClinicalTrials.gov, have not been registered in India, as required by law. PLoS One. 2020;15(6): e0234925. https://doi.org/10.1371/journal.pone.0234925
- Clinical Trials Registry – India. ICMR National Institute of Medical Statistics. CTRI Dataset and Description. Cited on 2023 June 30. Available from: http://ctri.nic.in/Clinicaltrials/CTRI_Dataset_and_Description.pdf
- Venugopal N, Saberwal G. A comparative analysis of important public clinical trial registries, and a proposal for an interim ideal one. PLoS One. 2021;16(5): e0251191. https://doi.org/10.1371/journal.pone.0251191
- National Library of Medicine. ClinicalTrials.gov. Cited on 2023 June 30. Available from: https://clinicaltrials.gov/
- ANZCTR: Australia New Zealand Clinical Trials Registry. Sydney (NSW): NHMRC Clinical Trials Centre, University of Sydney (Australia). Cited on 2023 June 30. Available from: https://www.anzctr.org.au/
- CRIS: The Clinical Research Information Service. Korea Disease Control and Prevention Agency. Cited on 2023 June 30. Available from: https://cris.nih.go.kr
- Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database – Update and key issues. N Engl J Med. 2011;364(9): 852–860. https://doi.org/10.1056/NEJMsa1012065
- House of Commons, UK Parliament, Science and Technology Committee. Research integrity: clinical trials transparency. 2018. Cited on 2023 June 30. Available from: https://publications.parliament.uk/pa/cm201719/cmselect/cmsctech/1961/1961.pdf
- Goldacre B. How to Get All Trials Reported: Audit, Better Data, and Individual Accountability. PLoS Med. 2015;12(4): e1001821. https://doi.org/10.1371/journal.pmed.1001821
- van Valkenhoef G, Loane RF, Zarin DA. Previously unidentified duplicate registrations of clinical trials: an exploratory analysis of registry data worldwide. Syst Rev. 2016;5: 116. https://doi.org/10.1186/s13643-016-0283-8
- Bruckner T, Vidal J. Access to clinical trial data in Europe. Lessons from EudraCT for Eudamed and the Clinical Trials Information System. Health Action International; 2021 [Cited on 2023 June 30]. Available: https://haiweb.org/wp-content/uploads/2021/09/Lessons-Eudamed-and-CTIS-2021.pdf
- Pandey A, Aggarwal A, Maulik M, Gupta J, Juneja A. Challenges in Administering a Clinical Trials Registry: Lessons from the Clinical Trials Registry-India. Phar Med. 2013;27: 83–93. https://doi.org/10.1007/s40290-013-0009-3
- Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated Trials in the ClinicalTrials.gov Results Database: Evaluation of Availability of Primary Outcome Data and Reasons for Termination. Briel M, editor. PLoS One. 2015;10: e0127242. https://doi.org/10.1371/journal.pone.0127242
- Chakraborty I, Shreya A, Mendiratta J, Bhan A, Saberwal G. An analysis of deficiencies in the ethics committee data of certain interventional trials registered with the Clinical Trials Registry–India. PLOS Glob Public Health. 2022;2: e0000617. https://doi.org/10.1371/journal.pgph.0000617
- De Vito NJ. Trial Registries for Transparency and Accountability in Clinical Research. University of Oxford; 2022.
- Viergever RF, Terry RF, Karam G. Use of data from registered clinical trials to identify gaps in health research and development. Bull World Health Organ. 2013;91: 416–425C. https://doi.org/10.2471/BLT.12.114454
- Tetteh O, Nuamah P, Keyes A. Addressing the quality of submissions to ClinicalTrials.gov for registration and results posting: The use of a checklist. Clin Trials. 2020;17: 717–722. https://doi.org/10.1177/1740774520942746
- Silverman E. NIH releases data showing more clinical trial sponsors are reporting results, but most miss deadlines. Cited on 2023 June 30. Available from: https://www.statnews.com/pharmalot/2023/03/29/nih-fda-studies-trials-transparency/
- Keyes A, Mayo-Wilson E, Nuamah P, Lalji A, Tetteh O, Ford DE. Creating a Program to Support Registering and Reporting Clinical Trials at Johns Hopkins University. Acad Med. 2021;96: 529–533. https://doi.org/10.1097/ACM.0000000000003806
- The UN Secretary-General’s high level panel on access to medicines. Promoting innovation and access to health technologies. 2016 [Cited on 2023 June 30]. Available from: www.unsgaccessmeds.org/s/UNSG-HLP-Report-FINAL-12-Sept-2016.pdf