ARTICLE
The marketing of OxyContin®: A cautionary tale
David S. Egilman, Gregory B Collins, Julie Falender, Naomi Shembo, Ciara Keegan, Sunil Tohan
DOI: https://doi.org/10.20529/IJME.2019.043Abstract
This paper provides a review of Purdue Pharma, LP’s development and marketing of the long-acting oral narcotic OxyContin®. Within five years of the drug’s launch, OxyContin® became the number-one prescribed Schedule II narcotic in the United States. This commercial success was in part the result of a marketing campaign that promoted questionably “distinctive” benefits and minimised the very real dangers of OxyContin®, which include abuse, addiction, overdose, and death. The marketing was based on scientifically invalid or unproven claims of safety and efficacy, inappropriate, off-label marketing, and inadequate warnings. When the FDA belatedly asked for changes to some of the marketing language, Purdue exploited these changes to further marketing objectives and misled healthcare practitioners. This case highlights questions of industry and governmental/regulatory accountability and responsibility for the production, marketing and sale of pharmaceutical products that increase risk while driving enhanced profits.
Introduction
The development and marketing of new drugs inevitably involves striking a balance between efficacy and safety for patients, and profit for the pharmaceutical company. Unfortunately, such a balance is not always achieved, and aggressive, even misleading, marketing by a drug company can put the health and safety of patients at undue risk. We review Purdue Pharma’s marketing of the long-acting oral narcotic OxyContin®. This history provides a cautionary tale of aggressive, profit-driven marketing of a dangerous drug with inadequate preclinical testing for safety; misleading warnings on the product label; unbalanced advertising; and regulatory compromise. The US Food and Drug Administration (FDA), although charged with protecting the public by monitoring the preclinical trials, marketing, advertising and promotion of new drugs, failed to protect the public from the deceptive marketing of a dangerous pharmaceutical product.
In the early 1990s, Purdue Pharma was faced with the imminent expiry of its patent on MS Contin®, a long-acting, (q12 hour) morphine-containing pain medication. Threatened with the spectre of competition from generics, the company developed OxyContin®, a slow-release version of oxycodone, as a new q12 hour pain reliever. The FDA approved OxyContin® in 1995. Purdue launched sales in 1996, marketing OxyContin® as the longest-acting narcotic pain reliever available. It promoted OxyContin® as a superior alternative to other pain medications such as Percocet and Percodan, which afford shorter periods of pain relief (4 to 6 hours.) (1). This marketing was incredibly successful. By 2000, US health professionals had written approximately 5.8 million OxyContin® prescriptions, and OxyContin® had become the number-one prescribed Schedule II narcotic in the United States (1, 2).
Unfortunately, this commercial success was at least in part the result of a marketing campaign that included off-label sales, promoted questionably unique benefits of the product, and minimised the very real dangers of OxyContin®, including abuse, diversion, addiction, overdose, and death (3). Since the drug’s introduction in 1996, opioid related deaths have increased dramatically. From 1990 to 2017, over 210,000 people have died in the US due to prescription opioid overdoses alone (4, 5).
A confidential justice department (DOJ) report from 2006 shows that Purdue Pharma was aware of the widespread abuse of OxyContin® and proceeded to conceal this information until it was leaked in 2017 to the journalist Barry Meier who published some parts of the investigatory record (4, 6). At the conclusion of a four-year-long investigation, the DoJ recommended that three Purdue Pharma executives be charged with felonies. This investigation provided an opportunity to expose the evidence against Purdue. Unfortunately, the George W Bush administration at the time did not support this indictment, and the DOJ allowed the Purdue executives to plead guilty to misdemeanors. More importantly, the DOJ sealed the investigatory record and Purdue’s misleading branding and marketing tactics were not made public. Instead, aggressive marketing led to increased prescription of Oxycontin®. From 2002 to 2009 the number of extended release opioid prescriptions increased 146%, from 9.3 to 22.9 million (7).
Unsupported claims of low addiction risk in chronic pain patients
As early as 1997, Purdue’s OxyContin® physician-directed promotional pieces, including advertisements, contained statements that “less than 1% of patients taking opioids actually become addicted” (8); that addiction to opioid medication is “rare”; and that the notion that “opioid addiction (psychological dependence) is an important clinical problem in patients with moderate to severe pain treated with opioids,” was a “myth” (9).
Purdue relied on three irrelevant documents to support its assertions (10, 11, 12). First, in one study, Medina and Diamond examined drug dependency among patients taking various medications for chronic headaches. They studied patients prescribed short-acting pain medications on an as-needed basis for relief of headaches (10). Only 62 of the 2,639 patients reviewed took narcotics, or a combination of analgesics and barbiturates, at least four times a week for at least six months (10). Of these 62 patients, 13 (19%) were dependent or abusers, based on definitions from the Diagnostics and Statistical Manual of Disorders (DSM) (13). Only two of the 62 had taken an oxycodone product – Percodan (5mg); neither took OxyContin®. This 19% rate of dependence and abuse is hardly “rare” and is certainly not “less than 1%” as Purdue asserted. Moreover, this abuse/addiction rate occurred in patients taking PRN (as needed) narcotics, which are much weaker (Schedule III) than OxyContin® (Schedule II). In fact, the original authors stated that “there is a danger of dependency and abuse in patients with chronic headaches” 10). When Diamond was asked about Purdue’s use of his study in a 2003 New York Times interview, he stated that “[Purdue’s characterization of addiction risk] distorts the picture and it clearly underplays the risk” (14).
In the second paper Purdue cited, Perry and Heidrich studied patients in pain undergoing burn debridement (11). They sent questionnaires to 151 burn facilities in the United States to review how burn pain was assessed and managed (11). Perry and Heidrich did not use any diagnostic criteria for addiction; nor did they collect patient data. Instead, the study was retrospective, relying entirely on the memory of staff members, and none of the surveyed doctors used OxyContin®, which was not on the market at the time of the study. Thus, the study provided no basis for comparison to current OxyContin® usage; nor could it support Purdue’s contention that pain patients treated with narcotics experience a low rate of addiction.
Finally, to support its low risk of addiction claims for OxyContin®, Purdue relied on a 110-word letter to the editor of the New England Journal of Medicine written by Porter and Jick in 1980 – 16 years before the introduction of OxyContin® (12). The letter, in its entirety, is as follows:
“To the Editor: Recently, we examined our current files to determine the incidence of narcotic addiction in 39,946 hospitalized medical patients who were monitored consecutively. Although there were 11,882 patients who received at least one narcotic preparation, there were only four cases of reasonably well documented addiction in patients who had no history of addiction. The addiction was considered major in only one instance. The drugs implicated were meperidine in two patients, Percodan in one, and hydromorphone in one. We conclude that despite widespread use of narcotic drugs in hospitals, the development of addiction is rare in medical patients with no history of addiction” (12).
There are several problems with Purdue’s reliance on this letter. First, this retrospective analysis does not include a single patient who was prescribed a controlled-release oxycodone product like OxyContin®. Later, in sworn testimony, Dr. Jick stated that, “We don’t have any information in this letter which relates to these “[people taking OxyContin®]” (15). Only 450 of the nearly 12,000 patients reviewed took an oxycodone product, and at least one of the four addiction cases identified involved a patient who took such a product: Percodan (15). The largest dose of oxycodone in Percodan is equivalent to the lowest recommended dose of OxyContin®.
As is the case with the other studies Purdue cited, the patients Porter and Jick studied bear little resemblance to those likely to be prescribed OxyContin®. Porter and Jick studied hospitalised patients who may have received as little as one narcotic pill or shot, generally used the drug for a brief time, and had the drug administered by hospital personnel. Porter and Jick did not follow patients after discharge (15). Thus, the study could not reliably detect addiction, which develops over time after repeated use; nor could it address the arguably higher addiction risk in unsupervised ambulatory outpatients, who are most likely to use a controlled-release opiate on an extended basis. The Porter and Jick study did not mention OxyContin® at all because it was completed twenty-five years before OxyContin® was sold. Most OxyContin® patients self-medicate at home, outside of the healthcare setting, where they can alter both the prescribed dose and dose schedule—alterations which increase addiction potential and rarely occur in the hospital setting. Much like Perry and Heidrich, Porter and Jick did not rely on standard DSM criteria to diagnose addiction, but instead based their results on the presence or absence of a written diagnosis of addiction in doctors’ or nurses’ notes in patient files (15, 16). This is likely to have resulted in an under-recording of the “addiction” diagnosis. With these glaring methodological flaws, Jick’s study should never have been used to support the contention that addiction in pain patients (including OxyContin® patients) is “rare.”
At the same time, Purdue failed to inform physicians of several studies that do indicate high rates of opioid addiction for chronic pain patients. In 1992, Fishbain et al found rates of addiction of 3.2%-18.9% in their meta-analysis of the scientific literature on chronic pain patients (17). These percentages were reiterated by Barry Dickinson in a 2000 review article for the American Medical Association Council on Scientific Affairs (18). A review of the literature on chronic pain and addiction reveals that numerous papers document rates of addiction between 3 and 34 percent in chronic pain patients (17, 19, 20, 21, 22, 23).
In 2001, the FDA concluded that the three references did not support Purdue’s assertion of low risk, and required the company to change OxyContin®’s patient package insert wording from “iatrogenic ‘addiction’ to opioids legitimately used in the management of pain is very rare” (24) to “data are not available to establish the true incidence of addiction” (25). Unfortunately, this FDA-ordered “correction” occurred years after it had approved the original wording, long after the original misleading label was in the hands of millions of patients and prescribers.
Despite this change, Purdue personnel continued to rely on at least one of these studies to assert that addiction to OxyContin® is “rare” (26). In 2003, Dr. Curtis Wright, Purdue’s Executive Director in Risk Assessment Coordination for New Products, testified that he still believed addiction to OxyContin® was rare (26). Dr. Wright based his opinion on the Agency for HealthCare Policy and Research (AHCPR) guidelines, which in turn relied on the Porter and Jick letter (26). Tellingly, the AHCPR guidelines themselves state that they are “no longer viewed as guidance for current medical practice” (27). Dr. Wright served as the FDA medical reviewer for OxyContin® prior to being hired by Purdue. His transition from OxyContin® FDA approval-reviewer to Purdue executive raises important questions about industry influence and the problems of the “revolving door” between government agencies and the industries they are supposed to regulate.
Inadequate warnings for addiction risk on patient package inserts
From 1997 to 2001, Purdue’s product labelling for OxyContin® failed to adequately warn about potential abuse or addiction. The company failed to include addiction warnings in the label’s “Warning,” “Precautions,” and “Information for Patients/Caregivers” sections; omitted contraindication warnings for people with prior drug addiction; and neglected to list any symptoms of opioid withdrawal (24, 28, 29, 30, 31).
The inadequacy of the OxyContin® warning label is underscored when compared to other addictive oral controlled-release opioid analgesics containing morphine sulphate. MS Contin (Purdue Frederick) and OraMorph SR (Roxanne) were similarly marketed as controlled-release 12-hour pain control products, in addition to being Schedule II drugs. At the time, the MS Contin label stated that “psychological and physical dependence may develop upon repeated administration” (32). The OraMorph SR® (Roxanne) label acknowledged the addiction risk even more clearly: “Morphine is the most commonly cited prototype for a narcotic substance that possesses an addiction-forming or addiction-sustaining liability. A patient may be at risk for developing dependence to morphine if used improperly or for overly long periods of time” (33). The OraMorph SR® label also alerted physicians that “Individuals with a history of opioid or other substance abuse or dependence, being more apt to respond to euphorigenic and reinforcing properties of morphine, would be considered to be at greater risk” (33).
Additionally, there were significant discrepancies between Purdue’s United States OxyContin label warnings and its foreign warnings for the same product. Unlike Purdue’s warning in the United States that suggested that OxyContin® can be used for moderate to severe pain, Mundipharma (a corporation tied to Purdue Pharma 34) in Austria stated that OxyContin® only be used for the “treatment of severe pain” (35). Additionally, the package insert in Austria differentiated between indications for non-malignant and cancer pain. For non-malignant pain the insert stated: “Treatment with OxyContin® should be brief and should be temporarily interrupted to minimize the risk of dependence” (35). The Austrian version also reiterated the risk of addiction in the “Side Effects” section, stating that “habit forming may occur in patients who are administered OxyContin®” (35).
Purdue’s osteoarthritis indication
Purdue also expanded the indications for Schedule II OxyContin® from the narrow market of malignancy sufferers to the much larger market of musculoskeletal pain sufferers. At the inception of the FDA approval process in the early 1990s, Purdue wanted FDA approval for OxyContin® use in patients with arthritis (36). But in March 1993, the FDA medical reviewer for OxyContin®, Dr. Curtis Wright (later Purdue’s Executive Director in Risk Assessment Coordination for New Products), alerted the Purdue OxyContin® project team that others at the FDA felt OxyContin® was not appropriate for patients suffering from osteoarthritis. Dr. Wright told Purdue that the FDA would not even approve a protocol designed to test the effectiveness of opioids in osteoarthritis treatment (26). Wright suggested that Purdue could overcome those FDA objections by re-writing their protocol to state that osteoarthritis patients were being used as pain models, not as target patients (26). Once the study was complete, Purdue circumvented the FDA’s initial negative position, and used it to market the drug for osteoarthritis.
In 1999, Purdue began promoting OxyContin® for arthritis sufferers in general. For example, one advertisement for OxyContin® had the slogan, “Proven Effective in Arthritis Pain” (37). The FDA took notice of a subsequent Purdue advertisement and reprimanded the company, writing, “…your journal ad is misleading because it suggests that OxyContin® can be used as a first-line therapy for the treatment of arthritis when such has not been demonstrated by substantial evidence…You should immediately discontinue the use of this journal advertisement” (38). However, in their 2001 Budget Plan, Purdue outlined a strategy to continue to promote OxyContin® to specialists in rheumatology, and to other practitioners dealing with arthritis and musculoskeletal pain (39).
Purdue’s “revised label” is still misleading
In July 2001, after receiving numerous reports of abuse and drug-related deaths, the FDA demanded that the drug company revise its insert language 40). Although Purdue complied with the FDA’s demands and revised its label, the changes created additional problems.
By 2002, the FDA and the media had become greatly concerned over the illicit use of OxyContin® (41). Purdue took advantage of this concern, and altered the label to make it appear as though illegal use and abuse were the only addiction-related problems associated with OxyContin® use: “Oxycodone can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing OxyContin® in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion” (42).
Purdue neglected to mention that the drug can be abused and can cause addiction in its intact orally-administered form, even when taken as prescribed. The new label stated:
“The development of addiction to opioid analgesics in properly managed patients with pain has been reported to be rare. However, data are not available to establish the true incidence of addiction in chronic pain patients” (25). These statements are contradictory: if data are not available, how can the rate of addiction be “reported to be rare”?
Purdue’s revised label tried to create an artificial distinction between physical dependence and addiction, stating, “Abuse and addiction are separate and distinct from physical dependence and tolerance” (42). This is not true. While drug tolerance and physical dependence without addiction may occur with pain treatment, these symptoms are also well-recognised diagnostic symptoms of opiate dependence (43). Resolving the question of whether addiction is present in a non-malignant pain patient presenting with drug tolerance, withdrawal, and physical dependence is not easy for the practising physician. Purdue conveyed a false sense of security that the non-malignant chronic pain patient who is ever-demanding of more and stronger narcotic medications is merely a predictable example of “pseudo-addiction” (inadequate dosing, leading to requests for stronger doses), innocuous tolerance, or the expected physical dependence on opiates (44). Physicians were thus discouraged from considering these demands as signs of “real” addiction, and instead were encouraged to increase the dose of OxyContin.
Purdue exploits the new FDA-approved label to expand its market
The FDA was concerned that Purdue had used its label to inappropriately broaden the indications for use of OxyContin® while understating the risks of abuse. After negotiations with the FDA, Purdue revised its OxyContin® label in 2002 to include a black box warning—the strongest warning found on prescription drugs—regarding abuse risks, and a new section on “Misuse, Abuse, And Diversion of Opioids” (42). The company saw the change as an opportunity to further “broaden OxyContin® Tablets usage in the management of pain,” with special emphasis on post-operative pain (45). The black box warning stated that OxyContin® should be restricted to patients “with moderate to severe pain when a continuous, around-the-clock analgesic is needed for an extended period of time” (25). Purdue’s 2002 Budget Plan explained how they intended to take advantage of this new language, “The action taken by the FDA to clarify the OxyContin® Tablet labelling has created enormous opportunities. In effect, the FDA has expanded the indication for OxyContin® Tablets to any patient with moderate to severe around the clock persistent pain…” [emphasis added]. This moved beyond post-operative patients to simply any patient with pain. The Budget Plan went on to proclaim that, “This broad labelling is likely to never again be available for an opioid seeking FDA approval. This may give OxyContin® Tablets a competitive advantage” 45). Thus, Purdue seized on the FDA label change to expand the market for OxyContin®, a Schedule II opioid narcotic, as a first line agent for the relief of moderate pain, a distinction previously reserved for Schedule III and IV narcotics and non- Scheduled alternatives like NSAIDS and Tramadol. This marketing strategy, promoted to prescribing physicians, further increased the risk of addiction for patients.
Purdue’s marketing campaign: downplaying addiction risk through AstroTurf
Purdue directed a great deal of its marketing directly to patients via brochures, videos, advertisements and the internet. It provided information to doctors and consumers through an apparently independent entity called “Partners Against Pain,” claimed to be an “alliance of patients, caregivers and health care providers”, but actually an “AstroTurf” or fake grass roots organisation. Purdue published and copyrighted the “Partners Against Pain” website, and the written materials on the site represented the interests and viewpoints of its owner. For example, a “Frequently Asked Questions” booklet available from the site, titled “A Guide to Your New Pain Medication and How to Become a Partner Against Pain,” reassures readers that OxyContin® only rarely presents an addiction risk (46).
One question asks, “Aren’t opioid pain medications like OxyContin® Tablets ‘addicting’? Even my family is concerned about this.” Purdue proffered the following misinformation as its answer:“Drug addiction means using a drug to get “high” rather than to relieve pain. You are taking opioid pain medication for medical purposes. The medical purposes are clear and the effects are beneficial, not harmful” (46). This “guide to patients” misleads patients into believing that their motivation for taking OxyContin® (ie, for pain instead of to “get high”) is the major determinant of whether they are, or will become, addicted to the medication. Yet, as is the case with all opiates, some patients will become addicted even if they initially used the drug to relieve pain and not to “get high.” There is no scientific support for the notion that the user’s motivation for initially using opiates determines whether he or she will become addicted later.
In 2001, in another question and answer section of the “Partners Against Pain” website, Purdue declared:
“When you feel pain, your pain is real… Remember: You have every right to ask [doctors and nurses] to help you relieve the pain as much as possible (47). By implying that patients have a right to as much pain relief as they wish without any regard for prescriber objections or for possible adverse reactions, Purdue encouraged patients to demand its drug, and propagated misinformation about pain perception and risk of addiction.
In 1986, the World Health Organization developed practice guidelines for cancer pain management and recommended that treatment begin with non-opioid analgesics (48). Purdue cited the WHO guidelines in their own marketing materials; however, Purdue also used the marketing “the one to start with,” misconstruing the WHO’s guidelines and positioning OxyContin® as the first drug of choice for pain (49, 50).
Purdue’s pamphlet and informational video, both titled, “From one pain patient to another,” encouraged patients to doctor-shop to find providers who were most willing to prescribe narcotics. Patients were told, “Don’t be afraid about the things you’ve heard about these drugs [opioids],” and, “…find the right doctor.” One “patient” featured in the video remarked, “I think it is very unfortunate that so many physicians are reluctant to treat people like me, who have moderate chronic pain, with opioids” (51). Another patient was shown being treated with 1200 mg of OxyContin® per day! Purdue thus disparaged the more cautious prescribing practices of responsible health practitioners who approached this drug as potentially addictive.
Starting on January 8, 2001, Purdue implemented a promotional letter campaign to physicians citing the Porter and Jick letter:
“The risk of addiction to opioids in clinical care has been greatly exaggerated. Experts in pain currently define addiction as, ‘… the compulsive use of a drug for non-medical purposes, usually with harm to the individual.’ Very few patients taking opioids for pain fit this description. In fact, a survey of more than 11,000 opioid using patients, taken over several years, found only four cases of documented addiction…it’s important to allay any fears patients may have about taking opioids” (52).
This is despite the fact that Dr. Jick had disavowed the relevance of his study to OxyContin® patients (15, 53).
Purdue also promoted OxyContin® as a substitute for less addictive, safer pain medications. In their 1997 Budget Plan they explained,
“When an opioid naïve patient needs an opioid analgesic, physicians are reluctant to begin them on MS Contin. Therefore, OxyContin® is the one to start with for patients who would otherwise be started on Percocet, Lortab, Vicodin or Tylenol #3” (54).
All of these drugs are narcotics, but none have the same addictive potential as OxyContin®, as they combine low doses of oxycodone or codeine with either aspirin or acetaminophen. Their dosing is limited by side effects of the anti-inflammatory or acetaminophen component of the pill, and they are less subject to abuse through pill crushing or chewing. Purdue’s marketing of OxyContin®, a single-entity narcotic with higher doses of 10, 20, 40, 80, or 160 (removed from market) milligrams of oxycodone as a preferred alternative to these lower strength narcotics, put patients at higher risk for addiction.
Purdue’s unbalanced ad campaign
Purdue’s questionable marketing strategy continued with unbalanced journal advertisements. An October 2002, an OxyContin® advertisement in the Journal of the American Medical Association (JAMA) stated, “When it’s time to consider q4-6h opioids…remember, effective relief takes just two” (55). Purdue failed to mention the abuse potential of opioids in the body of the advertisement. It did use some advertising space to explain a few of the side effects, including, “constipation, nausea, sedation, dizziness, vomiting, pruritus, headache, dry mouth, sweating and weakness,” but it did not mention abuse, addiction, diversion or death (55). A November 13, 2002 OxyContin® advertisement also featured in the JAMA showed a man and a boy fishing with the prominent headline, “There can be life with relief.” The ad also depicted two paper medication dosage cups labelled “8 AM” and “8 PM” 56). The ad conveys an image of a normal, pleasant life achieved through a simple twice-daily medication regimen, belying the addiction risks inherent in this powerful Schedule II narcotic drug. In keeping with this upbeat imagery, Purdue omitted information on OxyContin®’s abuse liability and the other statements contained in the FDA-imposed “black box warning” from the body of the ad.
In early 2003, the FDA sent Purdue a warning letter accusing the company of deceptive advertising, citing the JAMA advertisements:
“The typical physician reviewing an advertisement for a prescription drug would expect the most serious risks associated with the drug to be included in the body of the ad. The body of these ads contains no discussion of the potentially fatal risks associated with the drug and its potential for abuse. Moreover, expectation that the most relevant risks have been disclosed in the body, rather than the brief summary of your ads is exacerbated by having a statement in the body of your ads that begins ‘the most serious risk…’ implying that what follows is a complete statement of the drug’s most serious risks, not that there are other, more serious risks to be aware of. Therefore, the language in the body of your ads reinforces the impression that the most serious risks have been disclosed when in fact they have not. …the body of these ads contains no discussion of the potentially fatal risks associated with the drug and its potential for abuse” (57).
The letter went on to state:
“Your journal advertisements omit and minimize the serious safety risks associated with OxyContin®, and promote it for uses beyond which have been proven safe and effective…your journal advertisements fail to present in the body of the advertisement critical information regarding limitations on the indicated use of OxyContin®, thereby promoting OxyContin® for a much broader range of patients with pain than are appropriate for the drug” (57).Subsequently, Purdue published a retraction of its previous false claims and statements in advertisements in the June 18, 2003 issue of JAMA (58). Remarkably, it was once more able to turn its response to an FDA reprimand into a marketing advantage. The retraction page contained the black box warning effectively listing the drug as indicated for moderate pain (25). Purdue thus used its FDA “correction” to again promote its schedule II drug as a substitute for less dangerous, non-addictive drugs.
Misrepresentation of product features in marketing materials
Purdue has centred its promotional and marketing focus for OxyContin® on the q12h dosing schedule. When OxyContin® was first introduced, Purdue stated that OxyContin® offered a “significant advantage” because “unlike short-acting pain medications, which must be taken every 3 to 6 hours—often on an ‘as-needed basis,’ OxyContin® tablets are taken every 12 hours, providing smooth and sustained pain control all day and all night” (36).
Purdue’s 1998 OxyContin® Budget Plan describes the importance of q12 dosing to sales: “Our marketing research indicates that the most important feature of OxyContin® tablets, beyond the familiarity of oxycodone, is the q12h dosing schedule. In all seven pre-launch market research projects, healthcare professionals stated that this is the most compelling reason to prescribe the OxyContin® tablets” 59).
However, Purdue’s marketing materials omitted key information on efficacy. In various studies, OxyContin® did not consistently relieve pain for the entire 12-hour dosing interval and many patients required “rescue medication” — short acting oxycodone 60, 61, 62). On the other hand, some patients who have a delayed response may be overmedicated if they are dosed on a q12 basis (63). Instead of describing the wide range of patient responses and the importance of individual dose titration, to maintain its marketing “advantage,” Purdue told physicians that if their patients weren’t experiencing twelve hours of pain relief, they should increase the q12 dose as a response to pain breakthrough (64). This resulted in a much higher q12 dose escalation in some patients who instead should have received lower, more frequent dosing.
Purdue also encouraged dose escalation in a marketing piece for doctors called, “Counseling Your Patients and Their Families Regarding the Use of Opioids to Relieve Pain” (49). Doctors were instructed on how to answer questions from patients hesitant to accept opioid therapy. One question asked, “If I develop tolerance to this drug, what’s left for me to take when I really need pain relief?” Purdue suggested that a doctor might reply,
“Tolerance to opioids may occasionally occur. Usually all it takes to correct this situation is to increase the dose. Remember, opioids are not limited to a “maximum” dose as non-opioids are – an effective dose can be found for virtually any type or severity of pain” (49).
By encouraging ever-higher doses in response to breakthrough pain or increased drug tolerance, Purdue’s fixed q12 dosing recommendations created an addiction-generating machine.
Purdue knew the pain-relieving effect of OxyContin® was not fixed at 12 hours for all patients. During the 1999 patent trial, Dr. Robert F. Kaiko, OxyContin’s inventor, acknowledged that he had no evidence to support 12-hour efficacy (65). Its patent stated “a mean minimum plasma concentration from about 3 to about 30 ng/ml from a mean of about 10 to about 14 hours after repeated administration every 12 hours through steady-state conditions” (66). The half-life followed a Gaussian curve with half-lives ranging from 6-14 hours or more (67).
This point is further supported by the scientific data that is available on dose ranges. Sunshine et al. determined that the median effective dose interval for OxyContin® occurred at 10 hours (68). The OxyContin® instructions suggest using only a 12-hour dosing schedule. Most other pain medications provide a dosing frequency range to meet variable symptoms and metabolic rates. Purdue did not use a dose frequency range for OxyContin® for both patent and marketing reasons. The company understood that its patent protection for OxyContin®, and its subsequent success, relied in very large measure on OxyContin®’s 12 hour duration of effect, so Purdue had huge incentives not to present any other dosing schedule or frequency, and instead focused attention on increasing the dose itself to improve pain relief (69). Changing the dose, however, does not necessarily change a drug’s half-life, so, while an increased dose of OxyContin® may extend the length of time the drug is effective in some people, it does not ensure a full twelve hours of pain relief (70).
Purdue encouraged doctors to double the dose every twelve hours in patients who had breakthrough pain (71). However, the half-life of a drug is a function of the rate of elimination, which increases as the dose increases; higher drug concentrations increase the elimination rate (72). Thus, doubling the dose does not solve the breakthrough problem, but instead increases the likelihood of tolerance development and subsequent symptoms of withdrawal (73).
Even though increasing dosage was dangerous, Purdue continued to release increased pill doses. When released in December 1995, 40 milligrams was the highest dose pill. This was based on the representation that doses as high as 80 mg per day would be sufficient to treat >95% of patients (69). In 1996 the FDA approved a Purdue request to double the maximum pill dosage to 80 mg (160/day) (74). In 2000 the FDA approved another doubling to 160/mg (320/day) (75). (Purdue voluntarily withdrew the 160mg pill in 2001. It retains the right to reintroduce this dose)(76). The maximum available dose thus increased four-fold in four years.
Downplaying addiction
Purdue downplayed the addiction potential of OxyContin® by claiming that “delayed absorption is believed to reduce the abuse liability of a drug” (24). In the OxyContin® product brochure and in other materials, this assertion was expressed as “delayed absorption, as provided by OxyContin® tablets, is believed to reduce the attraction of a drug for abuse” (77).
A search of Medline failed to reveal any study that supported the claim that controlled-release opioids are any less likely to lead to addiction than immediate release opioids. However, the higher doses of the active drug in OxyContin® tablets increased their attractiveness for addicts. The “street value” of OxyContin® tablets, currently going for about a dollar per milligram. Clearly the higher dose tablets are much sought after, despite their “controlled release” (78)(78). Also, opioid addicts suffer debilitating physical and emotional symptoms when they go into withdrawal. They take what they can get, whether it is a controlled or immediate release drug, to relieve these symptoms. Addicts quickly learned to circumvent the “slow absorption” by crushing and chewing the pills. Ironically, Purdue’s previous long acting opioid, MS Contin, could not be misused this way (79).
Moreover, Purdue reported that its OxyContin® formulation had a biphasic release, including a rapid onset analgesic phase in addition to its long-acting phase (31). Even if “slow-onset” reduces the risk of addiction, Purdue’s “biphasic” OxyContin® formulation did not provide this protection (80). If the speed of absorption affects the addiction potential, then OxyContin®’s fast release component would presumably increase the risk of addiction. Furthermore, if this claim is accurate, Purdue not only provides a claimed “slow release” oxycodone (OxyContin®), but also markets “rapid release” OxyIR and OxyFast which are often used off label as breakthrough medication in conjunction with OxyContin® (61, 81). If OxyContin® had a lowered addiction risk because of its controlled release, then these fast-acting opioids present a higher addiction potential. Purdue, however, issued no warnings regarding elevated addiction risk for OxyIR or OxyFAST.
Alcohol drug interactions
When oxycodone is mixed with alcohol, the short-term effects on the body can be extremely damaging and dangerous. The exposure of extended-release oxycodone in the body is increased heavily when administered with ethanol (82). Purdue’s warning label revised in 2015 only cites possible drug interactions of oxycodone with CNS depressants and mixed agonist drugs (71). The thirty-nine-page long warning packet fails to caution patients against ingesting alcohol when they have been prescribed OxyContin®. Also, ingestion of grapefruit juice—commonly ingested with alcohol—can “increase plasma concentrations and clinical effects of oxycodone”(83). Consumption of grapefruit juice can lead to increased side effects from OxyCodone, such as nausea, itch, and dizziness. Being that grapefruit juice inhibits the enzyme which mediates the “first-pass metabolism of oxycodone,” when paired with the drug grapefruit increases the concentration and the clinical effects (83). Again, Purdue neglected to warn patients about this danger.
Teenagers at parties may often be unaware of the effect that oxycodone can have on their system when combined with alcohol. An example of this phenomenon occurred in 2016. Jack Granger and his 19-year old brother, Nick, died after hosting a party (84). Toxicology results indicated that they died from a combination of oxycodone and alcohol (85).
How OxyContin® co-opted and subverted the FDA regulatory process
This review has highlighted the marketing strategy of a drug manufacturer that had drawn multiple citations and warnings from the FDA. Purdue exploited the FDA’s citations, none of which was accompanied by fines, by using them to further marketing objectives and mislead health practitioners about the addictive potential of Oxycontin®. After FDA intervention, the company continued to omit important information from the OxyContin® label, and distributed marketing materials that misrepresented the purported benefits and greatly minimized the risks of the drug. The OxyContin® chronicle indicates the many and varied ways in which a pharmaceutical manufacturer can undermine and compromise the regulatory process to the detriment of consumer safety. Many economists have noted that over time, regulatory agencies become “captured” by the industries they regulate (86). The revolving door marketing strategy that encourages pharmaceutical companies to hire FDA regulators was clearly exploited in the case of OxyContin®. This is not a novel phenomenon. As Janis noted, collective decision making is often accompanied by “Groupthink”: a process that encourages a “go along to get along” attitude (87).
Why Purdue’s marketing of OxyContin® worked
Purdue’s marketing of OxyContin® to doctors was aggressive. The 2019 formal complaint lodged against Purdue by the state of Massachusetts’s Attorney General revealed that the company “tracked Massachusetts doctors’ prescriptions, visited their offices, bought them meals, and asked them to put specific patients on Purdue drugs” (88). It selectively targeted doctors that they deemed most likely to change their prescribing habits to benefit OxyContin® prescribing amounts. Purdue “staff told the [owners of Purdue] that they would increase the number of sales visits and had hired McKinsey to study how to get doctors to prescribe more OxyContin.” Company marketing pieces disguised as scientific knowledge were sent to prescribers . For example, the piece Focused and Customized Education Topic Selections in Pain Management “falsely told doctors and patients that signs of addiction are actually ‘pseudoaddiction,’ and that doctors should respond by prescribing more opioids.” Purdue paid doctors nearly $100,000 to promote its opioids, while assuring them that OxyContin® was safe. Purdue has also spread false information to the academic sphere. A 2016 New York Times article cited one scientist in favor of prescribing opioids, but that scientist was funded by the owners of Purdue (89). In these ways it is clear how Purdue’s marketing worked to both deceive doctors and take advantage of their profit-seeking ways. And research shows that “marketing to physicians can be both informative and persuasive”—in other words, drug marketing to doctors works well to increase prescriptions (90). Purdue took advantage of this potential when marketing OxyContin®.
Conclusion
The OxyContin® case raises important questions of industry accountability and responsibility in the production, marketing and sale of potentially dangerous drugs and narcotics. Proper, appropriate, balanced, complete, and scientifically valid information must be in the hands of physician “learned intermediaries.” Direct to consumer marketing can circumvent physician judgment and cautious risk-avoidance in medication prescribing. Purdue Pharma, in its relentless zeal for sales growth and profits, abdicated its responsibility by subverting governmental regulatory authority, and by repeatedly and systematically misinforming prescribing doctors and the public about the characteristics and inherent risks of its Schedule II narcotic, OxyContin®. This case demonstrates that physicians who prescribe narcotics for pain need to be vigilant for self-serving, invalid, or unbalanced claims in their review of advertisements and corporate sales promotions for drugs, especially those, like OxyContin®, with potential for abuse, diversion, addiction, overdose, and death.
Companies have an obligation to stockholders to maximise profits by increasing revenues and by minimising or avoiding costs, including social costs and the costs of adverse reactions to their products. They are not self-regulating entities (91). Physicians need to be aware that these business imperatives can drive marketing practices which are not scientifically or ethically based, and which may be based on flawed or untested clinical assumptions.
Statement of Conflict of Interest
DE has consulted on opioid litigation at the request of addicted patients and counties. GC has consulted on opioid litigation at the request of addicted patients. DE is the owner of Never Again Consulting; NS, CK, and ST were compensated by Never Again Consulting for their contributions.
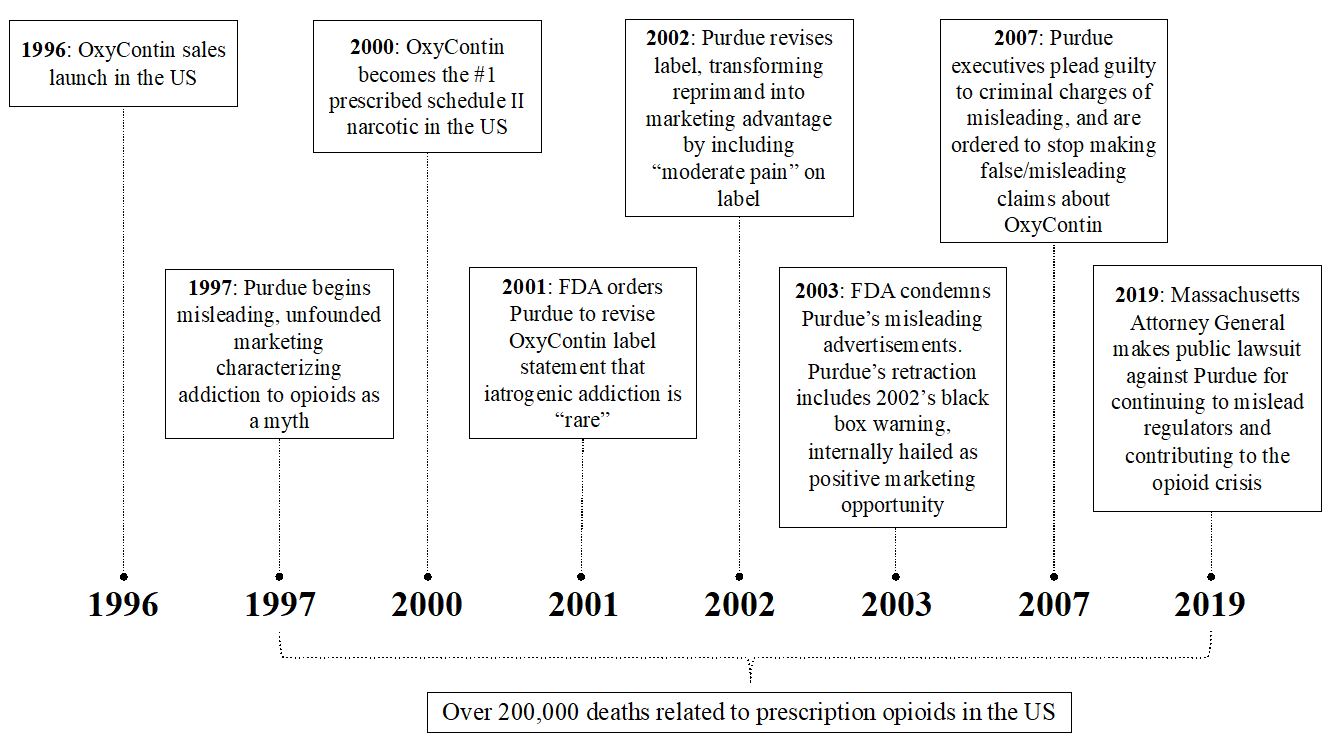
Appendix 1: Timeline of Events (5)
References
- United States Drug Enforcement Administration. Prescription Drug Trafficking & Abuse Trends. 2013 May 15[cited 2019 Mar 10]. Available from: https://www.deadiversion.usdoj.gov/pubs/presentations/euus2013.pdf.
- Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009 Feb; 99(2), 221-7. doi: 10.2105/AJPH.2007.131714
- American Addiction Centers. Dangers of Snorting or Injecting OxyContin. 2019 Jun 11[cited 2019 Mar 11].. Available from: https://americanaddictioncenters.org/prescription-drugs/smoking-snorting-injecting
- Meier B. Origins of an epidemic: Purdue Pharma knew its opioids were widely abused. New York Times. 2018 May 29[cited 2019 Mar 10]. Available from https://www.nytimes.com/2018/05/29/health/purdue-opioids-oxycontin.html
- Centers for Disease Control and Prevention. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC; 2017 Jun 14 [cited 2019 Mar 10]. Available from https://catalog.data.gov/dataset/wide-ranging-online-data-for-epidemiologic-research-wonder
- Meier B. Pain killer. Emmaus, Pa.: Rodale. 2003.
- Alam A, Juurlink DN. The prescription opioid epidemic: an overview for anesthesiologists. Can J Anesth. 2016 Jan; 63(1), 61–8. Doi:10.1007/s12630-015-0520-y.
- Purdue Pharma. I got my life back. Marketing Brochure. 1997. Norwalk, CT: Purdue Pharma. Date unknown [cited 2019 Mar 10]. Available from: https://repository.library.brown.edu/studio/item/bdr:927752/
- Purdue Pharma. News & Media. Pharmaceutical & Healthcare News from Purdue Pharma LP. Available from www.purduepharma.com/news-media/common-myths-about-OxyContin/”
- Medina JL, Diamond S. Drug dependency in patients with chronic headaches. Headache.1977 Mar;17(1):12–14. doi:10.1111/j.1526-4610.1977.hed1701012.x.
- Perry S, Heidrich G. Management of pain during debridement: A survey of U.S. burn units. Pain 1982 Jul;13(3), 267-80.
- Porter J, Jick H. Addiction rare in patients treated with narcotics. New Engl J Med. 1980 Jan 10; 302 (2):123.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. Washington, DC: APA;1968 [cited 2019 Mar 10]. <"a href=Available from https://www.madinamerica.com/wp-content/uploads/2015/08/DSM-II.pdf"> https://www.madinamerica.com/wp-content/uploads/2015/08/DSM-II.pdf
- Meier B. The delicate balance of pain and addiction. New York Times. 2003 Nov 25 [cited 2019 Mar10]. Available from www.nytimes.com/2003/11/25/science/the-delicate-balance-of-pain-and-addiction.html
- Jick H. Videotaped deposition of Herschel Jick.2002 Sep 19 [cited 2019 Mar 11]. 34, 19-20. Available from https://repository.library.brown.edu/studio/item/bdr:841737/
- American Psychiatric Association. Mental Disorders: Diagnostic and Statistical Manual. 1st ed. Washington, DC: The Committee on Nomenclature and Statistics, APA. 1952.
- Fishbain DA., Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clin J Pain. 1992 Jun; 8(2): 77–85.doi:10.1097/00002508-199206000-00003.
- Dickinson BD, Altman RD, Nielsen NH, Williams MA. Use of opioids to treat chronic, noncancer pain. West J Med. 2000 Jan;172(2):107–15. doi:10.1136/ewjm.172.2.107.
- Kouyanou K, Pither CE, Wessely S.et al. Medication Misuse, Abuse and Dependence in Chronic Pain Patients. J Psychosom Res. 1997 Nov;43(5): 497–504.doi:10.1016/s0022-3999(97)00171-2.
- Hoffmann NG, Olofsson O, Salen B, Wickstrom L. Prevalence of abuse and dependency in chronic pain patients. Int J Addict. 1995 Jun;30(8):919-27. doi:10.3109/10826089509055820.
- Ballantyne JC. Assessing the prevalence of opioid misuse, abuse, and addiction in chronic pain. Pain. 2015 Apr;156(4):567-8.doi: 10.1097/j.pain.0000000000000105
- Maruta T, Swanson DW, Finlayson RE. Drug abuse and dependency in patients with chronic pain. Mayo Clin Proc. 1979 Apr; 54(4):241–4.
- Maruta T, Swanson DW. Problems with the use of oxycodone compound in patients with chronic pain. Pain.1981 Dec;11 (3):389–96.doi:10.1016/0304-3959(81)90638-2.
- Physicians’ Desk Reference. OxyContin® Package Insert. 51st, 2166. 1997. Montvale, NJ, Thompson PDR.
- Physicians’ Desk Reference. OxyContin® Package Insert. 57th, 2852-2853. 2003. Montvale, NJ, Thompson PDR.
- Wright C. Deposition. 88-94. 2003 Jul 25 [cited 2019 Mar 10]. Available from: https://repository.library.brown.edu/studio/item/bdr:927750/
- The Agency for Healthcare Research and Quality. Acute Pain Management: Operative or Medical Procedures and Trauma Clinical Practice. Guideline No. 1. Rockville, MD: AHCPR;1992 Feb.
- Physicians’ Desk Reference. OxyContin® Package Insert. 52nd. 1998. Montvale, NJ, Thompson PDR.
- Physicians’ Desk Reference. OxyContin® Package Insert. 53rd. 1999. Montvale, NJ, Thompson PDR.
- Physicians’ Desk Reference. OxyContin® Package Insert. 54th, 2539. 2000. Montvale, NJ, Thompson PDR.
- Physicians’ Desk Reference. OxyContin® Package Insert. 55th. 2001. Montvale, NJ, Thompson PDR.
- Physicians’ Desk Reference. MS Contin Package Insert. 51st, 2150. 1997. Montvale, NJ, Thompson PDR.
- Physicians’ Desk Reference. OraMorph Package Insert. 51st, 2360. 1997. Montvale, NJ, Thompson PDR.
- Purdue Pharma. Global Network. Available from: https://www.purduepharma.com/partnering-with-purdue/pharma-capabilities/global-network/
- Austria-Codex Fachinformation. 2004. Wien, Österreichischer Apotheker. Available from https://repository.library.brown.edu/studio/item/bdr:841750/
- Purdue Pharma. New hope for millions of Americans suffering from persistent pain: Long-Acting OxyContin® Tablets now available to relieve pain. PR Newswire. 1996 May 31[cited 2019 Mar 11}. Available from: http://documents.latimes.com/oxycontin-press-release-1996/
- Purdue Pharma. Proven effective in arthritis pain. 2000 May 4 [cited 2019 Mar 11]. Available from https://repository.library.brown.edu/studio/item/bdr:841754/
- Salis S. FDA Warning Letter to Beth Connelly, Senior Associate, Regulatory Affairs, Purdue Pharma LP. 2000 May11[cited 2019 Mar 10]. Available from: https://repository.library.brown.edu/studio/item/bdr:841751/
- Purdue Pharma. 2001 Budget Plan: OxyContin® Tablets. 4-51. 2001[cited 2019 Mar 11]. Available from: https://www.documentcloud.org/documents/4490150-OxyContin2001.html?embed=true&pdf=true&responsive=false&sidebar=false&text=true
- Food and Drug Administration. OxyContin Information: FDA strengthens warnings for OxyContin®. 2001 Jul 25[cited 2019 Mar 10]. Available from:
- Meier B. OxyContin deaths may top early count. New York Times. 2002 April 15[cited 2019 Mar 11]. Retrieved from https://www.nytimes.com/2002/04/15/us/oxycontin-deaths-may-top-early-count.html
- Physicians’ Desk Reference. OxyContin® Package Insert. 56th, 2914. 2002. Montvale, NJ, Thompson PDR.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington DC: The Committee on Nomenclature and Statistics, APA; 1994.
- Weissman DE, Haddox JD. Opioid pseudoaddiction–an iatrogenic syndrome. Pain. 1989 Mar;36(3):363-6.
- Purdue Pharma. 2002 Budget Plan: OxyContin® Tablets. 1-47. 2002 [cited 2019 Mar 11]. Available from https://www.documentcloud.org/documents/4490164-OxyContin2002.html?embed=true&pdf=true&responsive=false&sidebar=false&text=true
- Purdue Pharma. A guide to your new pain medication and how to become a partner against pain. Date unknown [cited 2019 Mar 11]. Available from https://repository.library.brown.edu/studio/item/bdr:841749/
- Purdue Pharma. How you can be a partner against pain and gain control over your own pain. 2001 [cited 2019 Mar 12]. Available from https://repository.library.brown.edu/studio/item/bdr:841738/
- World Health Organization. Cancer Pain Relief. Geneva, Switzerland: WHO;1986 [cited 2019 Mar 10]. Available from https://apps.who.int/iris/handle/10665/37896
- Purdue Pharma. Counseling Your Patients and Their Families Regarding the Use of Opioids to Relieve Pain. Partners Against Pain. Norwalk, CT. 1997 [cited 2019 Mar 11]. Available from https://repository.library.brown.edu/studio/item/bdr:841753/
- United States General Accounting Office. Prescription Drugs: OxyContin® Abuse and Diversion and Efforts to Address the Problem. 2003 Dec [cited 2019 Mar 11]. Available from https://www.gao.gov/htext/d04110.html
- Purdue Pharma. From one pain patient to another. Purdue Pharma Promotional Video and Pamphlet. Norwalk, CT. 2000 [cited 2019 Mar 11]. Available from: https://repository.library.brown.edu/studio/item/bdr:841752
- Purdue Pharma. Promotional Letter.1998 Jan 8[cited 2019 Mar 11]. Retrieved from https://repository.library.brown.edu/studio/item/bdr:927753/
- Testimony of David Haddox on behalf of Purdue Pharma before the Sub-committe on Criminal Justice, Drug Policy, and Human Resources of the Committee on Government Reform. Exhibit B-3. 2004 Sep 2 [cited 2019 Mar 11]. Available from https://repository.library.brown.edu/studio/item/bdr:841747/
- Purdue Pharma. 1997 Budget Plan: OxyContin® Tablets. 2-36-2-26. 1997 [cited 2019 Mar 10]. Available from https://www.documentcloud.org/documents/4490123-OxyContin1997.html?embed=true&pdf=true&responsive=false&sidebar=false&text=true
- Purdue Pharma. Effective relief takes just two. JAMA: The Journal of the American Medical Association. 2002 Oct [cited 2019 Mar 10]. Available from: https://repository.library.brown.edu/studio/item/bdr:927751/
- Purdue Pharma. There can be life with relief. JAMA: The Journal of the American Medical Association. 11-06-2002 [cited 2019 Mar 11]. Available from https://repository.library.brown.edu/studio/item/bdr:927749/
- Abrams T. FDA Warning Letter. to Michael Friedman, CEO, Purdue Pharma. 2003 Feb 3 [cited 2019 Mar 11]. Available from https://repository.library.brown.edu/studio/item/bdr:841746/
- Reder R. Important correction: Letter to health care practitioners. JAMA: The Journal of the American Medical Association. 2003 Jul 18.
- Purdue Pharma. 1998 Budget Plan. 4-41. 1998 [cited 2019 Mar 10]. Available from https://www.documentcloud.org/documents/4490129-OxyContin1998.html?embed=true&pdf=true&responsive=false&sidebar=false&text=true
- Hagen NA, Babul N. Comparative clinical efficacy and safety of a novel controlled-release Oxycodone formulation and controlled-release hydromorphone in the treatment of cancer pain. Cancer.1997 Apr 1;79(7):1428-37.
- Citron ML, Kaplan R, Parris WCV, Croghan MK, Herbst LH, Rosenbluth RJ. Reder RF, Slagle NS, Buckley BJ, Kaiko RF. Long-term administration of controlled-release oxycodone tablets for the treatment of cancer pain. Cancer Investigation. 1998;16(8):562-71.
- Kaplan R, Parris WC, Citron ML, Zhukovsky D, Reder RF, Buckley BJ, Kaiko RF. Comparison of controlled-release and immediate-release oxycodone tablets in patients with cancer pain. J Clin Oncol. 1998; 16:3232-3.
- McGreal C. The making of an opioid epidemic. The Guardian. 2018 Nov 8 [cited 2019 Mar 12]. Available from: https://www.theguardian.com/news/2018/nov/08/the-making-of-an-opioid-epidemic
- Purdue Pharma. Professional Sales Aid, Artwork No. C5180.1999 May 25[cited 2019 Mar 11]. Available from https://repository.library.brown.edu/studio/item/bdr:841742/
- Kaiko R. Deposition. 10-22-1999. Retrieved from https://repository.library.brown.edu/studio/item/bdr:841736/
- Google Patents. US20100092570A1 – Controlled release oxycodone compositions. Retrieved from https://patents.google.com/patent/US20100092570A1/en
- Sangeetha, S. Pharmacokinetics: basic considerations— Plasma drug concentration‐time profile plotting data. Date unknown [cited 2019 Mar 11]. Available from: http://www.srmuniv.ac.in/sites/default/files/files/PharmacokineticsBasicConsiderations.pdf
- Sunshine A, Olson NZ, Colon A, Rivera J, Kaiko RF, Fitzmartin RD, et al. Analgesic efficacy of controlled-release oxycodone in postoperative pain. J Clin Pharmacol. 1996;36: 595-603.
- Stein, SH. Purdue Pharma LP against Endo Pharmaceuticals v. Euroceltique S.A., Opinion and Order. 16, 40. 7-5-2004. New York, New York, US District Court: Southern District of New York.
- Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011 Apr 11;171(7):686–691. doi:10.1001/archinternmed.2011.117
- Food and Drug Administration. Highlights of OxyContin® Prescribing Information. 2015 Aug [cited 2019 Mar 11]. Available from http://app.purduepharma.com/xmlpublishing/pi.aspx?id=o
- Birkett D. Pharmacokinetics made easy 11 Designing dose regimens. Australian Prescriber. 1996 Jul; 19(3), 76-8. doi:10.18773/austprescr.1996.069
- National Pain Centre. Opioid Tapering Information for patients. Date unknown [cited 2019 Mar 10]. Available from: http://nationalpaincentre.mcmaster.ca/documents/Opioid%20Tapering%20Patient%20Information%20(english).pdf
- New FDA Approved Drugs. OxyContin (Oxycodone HCl controlled-release). Centerwatch.com. 1996 May [cited 2019 Mar 11]. Available from https://www.centerwatch.com/drug-information/fda-approved-drugs/drug/120/oxycontin-oxycodone-hcl-controlled-release
- Long-acting OxyContin 174 tablets now available in 160 mg strength to relieve persistent pain. 2000 Jul 9 [cited 2019 Mar 11]. Available from https://www.eurekalert.org/pub_releases/2000-07/PN-LOtn-0907100.php
- US Dept of Justice, Diversion Control Division. Answers to Frequently Asked Questions Regarding OxyContin. Date unknown [cited 2019 Mar 11]. Available from: https://www.deadiversion.usdoj.gov/drug_chem_info/oxycodone/oxycontin_faq.htm#7.
- Purdue Pharma. Purdue Pharma Product Data Brochure.2000 Mar 27[cited 2019 Mar 11]. Available from: https://repository.library.brown.edu/studio/item/bdr:841739/
- Connecticut Clearinghouse. OxyContin / Oxycodone. Date unknown [cited 2019 Mar 11]. Available from https://www.ctclearinghouse.org/topics/oxycontin-oxycodone/ https://www.ctclearinghouse.org/topics/oxycontin-oxycodone/
- Welch JS. Letter to the Editor: Risk of intravenous abuse of oral morphine sulfate. Am Fam Physician. 1997 Oct 1;56(5):1307- 8.
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003 Apr 1;69(3):215-32.
- Purdue Pharma. 24 Hours of Oxycodone Pain Control – The Old Way- The New Way. 16-17. 1999 May 25[cited 2019 Mar 11] Available from: https://library.brown.edu/studio/item/bdr:841742/
- Malhotra BK. Matschke K, Wang Q, Bramson C, Salageanu J. Effects of ethanol on the pharmacokinetics of extended-release oxycodone with sequestered naltrexone (ALO-02). Clin Drug Investig. 2015 Apr;35(4):267-74. doi: 10.1007/s40261-015-0278-6.
- Nieminen TH, Hagelberg NM, Saari TI, Neuvonen M, Neuvonen PJ, Laine K, Olkkola K T. Grapefruit juice enhances the exposure to oral oxycodone. Basic Clin Pharmacol Toxicol. 2010 Oct; 107(4), 782–788. 2010. https://doi.org/10.1111/j.1742-7843.2010.00582.x
- Sheckler C. Charges filed in connection with party where Granger brothers overdosed. South Bend Tribune. 2015 Jun 22[cited 2019 Mar 11]. Available from: https://www.southbendtribune.com/news/publicsafety/charges-filed-in-connection-with-party-where-granger-brothers-overdosed/article_dc4abebc-2fba-11e5-a3ec-ff9ce46db843.html
- Sheckler, C. Coroner confirms cause of death in Granger brother overdoses. South Bend Tribune. 2015.
- Viscusi WK, Vernon JM, Harrington JE. Economics of Regulation and Antitrust. 3rd ed. Cambridge, MA: MIT Press; 2000.
- Janis IL. Groupthink: psychological studies of policy decisions and fiascoes. 2nd ed. Boston: Houghton Mifflin;1983. 351 pp. p.xii.
- Commonwealth of Massachusetts v. Purdue Pharma L.P. First Amended Complaint and Jury Demand. 2019 Jan [cited 2019 Mar 11]. Available from: https://www.mass.gov/files/documents/2019/01/31/Massachusetts%20AGO%20Amended%20Complaint%202019-01-31.pdf
- Goodnough A, Tavernise S. Opioid prescriptions drop for first time in two decades. New York Times. 2016 [cited 2019 Mar 11}. Available from: https://www.nytimes.com/2016/05/21/health/opioid-prescriptions-drop-for-first-time-in-two-decades.html
- LaPlante A. Marketing to Physicians Reaps Higher Returns for Drug Companies. Insights by Stanford Business. 2006 [cited 2019 Mar 11]. Available from: https://www.gsb.stanford.edu/insights/marketing-physicians-reaps-higher-returns-drug-companies
- Friedman M. The social responsibility of business is to increase its profits. New York Times Magazine. 1970 Sep 13 [cited 2019 Mar 12]. Available from: https://www.nytimes.com/1970/09/13/archives/article-15-no-title.html