RESEARCH ARTICLE
Surfactants and the importance of informed consent: Nurturing culturally competent care in healthcare settings
Priyanka Gupta, Vishwajeet Singh, Prince Pareek
DOI:10.20529/IJME.2024.042Abstract
Background: Culturally competent healthcare improves patient satisfaction and clinical outcomes. Many drugs, dressings and implants have human or animal-derived content which may conflict with patients’ religious beliefs, and may even have medicolegal implications.
Methods: This cross-sectional study (anonymous web-based survey) was done to understand the informed consent process followed by paediatricians and neonatologists in India, their views regarding disclosure pertaining to the animal origin of exogenous surfactants to patients’ families, and their willingness and ability to provide alternative surfactants based on parental preferences.
Results: A total of 114 neonatologists/paediatricians involved in neonatal care and using surfactants in their practice responded to the survey. Although 61(53.5%) neonatal care units stocked two or more brands of surfactant in their inventory, only 38(33.3%) units had both bovine and porcine preparations. Most (104, 91.2%) of the doctors always take parental consent before administering surfactants; but only a few (12,10.5%) said they always inform parents about its animal origin. None of the respondents offer parents a choice between bovine or porcine-origin surfactants, most (73, 64%) presuming that it would be irrelevant for the parents. However, many respondents (27, 23.7%) mentioned that they want to offer the choice to parents but are unable to do so because they do not stock both bovine and porcine preparations.
Conclusion: Although most parents might agree to a life-saving medicine in emergency situations, this does not mean they do not want to be informed. Healthcare professionals should not have a dismissive attitude to parental belief systems. They must use the antenatal period to take the cultural/spiritual history and the necessary consent.
Keywords: culturally competent care, informed consent, religion, surfactant
Introduction
A stable doctor-patient relationship is grounded in the principles of good communication, trust, respect, confidentiality and professional honesty. In the history of mankind, health providers and religious guides were often the same people. However, with the emphasis on a scientific approach in modern medicine, over time there has been a slow separation between medicine and spirituality. Patients’ religious and spiritual beliefs often come into play while making medical decisions. This indicates the need for healthcare providers and institutions to be culturally sensitive and respectful of patients’ religious and spiritual beliefs. Culturally competent healthcare needs to meet not only the patients’ medical needs but also the cultural, social, and religious needs of patients and their families. An opportunity should be provided to patients to discuss their views so as to tailor investigations and treatment to their belief system. This has the potential to improve patient satisfaction as well as clinical outcomes [1, 2, 3].
There are many drugs, dressings and implants with human or animal-derived content with potential conflicts with the religious beliefs of the patient and their family. An alternative to these may or may not exist [4, 5, 6]. Physicians need to be aware of the origin of these products, otherwise they may fail to explain this to their patients. Sometimes, the physician does not consider this issue pertinent and thus the patient and family are not made aware of it. This information may even be hidden intentionally due to concerns about conflicts between religious beliefs and the required treatment. The authors believe that in the Indian multicultural society with diverse faiths, the health practitioner cannot afford to ignore this pertinent issue. There are always medicolegal implications to ethical issues surrounding patient care and the physician should have sufficient knowledge of the ingredients of drugs and implants, as well as the religious considerations of treatment regimens. Patients’ and families’ autonomy and beliefs must be respected, and they must be given sufficient information in a comprehensible manner so that they can make informed decisions.
Over the past few decades, surfactants have played a crucial role in saving the lives of preterm babies in neonatology practice. When babies are born too early, their lungs often don’t produce enough natural surfactant leading to breathing difficulties. Surfactants are special substances that help keep the lung alveoli open, making it easier for these preterm babies to breathe. These natural surfactants are animal in origin, derived from calf or pig lung [7, 8, 9]. Due to lack of survey data among prospective Indian parents, it is an assumption that most parents would agree to a life-saving medicine in an emergency situation. However, such consent or choice should not be presumed in a country like ours with various religions, castes and faiths, where this could raise serious issues. Families still need to make their own informed decisions.
Parents of some premature infants might have concerns about the animal origin of this drug either on religious or safety grounds. Muslim or Jewish parents might object to the use of porcine surfactant, while Hindu parents could object to the use of bovine surfactant. Vegan or vegetarian parents might have concerns about the use of either surfactant [10, 11, 12]. This is a hard choice to make, especially in a country where surfactant availability itself is limited and most of the neonatal care units stock only one or the other type of surfactant. One may argue that an explanation regarding the animal origin of a surfactant may burden families with an additional dilemma when they are already grappling with complex decisions, but an informed choice is an ethical necessity.
To the best of our knowledge, the practices of paediatricians and neonatologists in India regarding the informed consent procedure during exogenous surfactant administration in a neonate have never been investigated. This survey was done to learn about the practice of informed consent followed by paediatricians and neonatologists across India, their views regarding disclosure to parents of the animal origin of exogenous surfactants, and their willingness and ability to provide alternative surfactant preparations based on parental preferences.
Methods
This was a cross-sectional study conducted during April-May, 2023, with due approval from the Institutional Ethics Committee (Ref. EC File No: 134 X/11/13/2023- IEC/22 dated April 18, 2023). An anonymous web-based survey was shared with 239 paediatricians/neonatologists across all states and Union territories of India, of which 114 responded ie, 47.7% response rate to the survey.
The questionnaire developed was validated by pilot testing it first on five paediatricians, whose data was excluded from the analysis. The paediatricians not using surfactants in their clinical practice and not involved in neonatal care were requested to skip the survey. The questionnaire recorded a bio-demographic profile including age, gender, place of work, details of clinical training and clinical experience of the respondents. The questionnaire had both open and closed-ended questions so as to understand the informed consent procedures followed by respondents while administering the exogenous surfactant.
The data was compiled, anonymised and analysed with SPSS (Version 23.0) software using descriptive statistics. Frequencies and proportions were used to summarise categorical variables. Mean, standard deviation (SD), median, quartiles and range were used to summarise quantitative variables. The Pearson Chi-square test was applied to study the possible association of age, gender, special training in neonatology, duration of clinical experience and place of work with the practice of taking consent and informing parents about the animal origin of surfactant during its administration.
Results
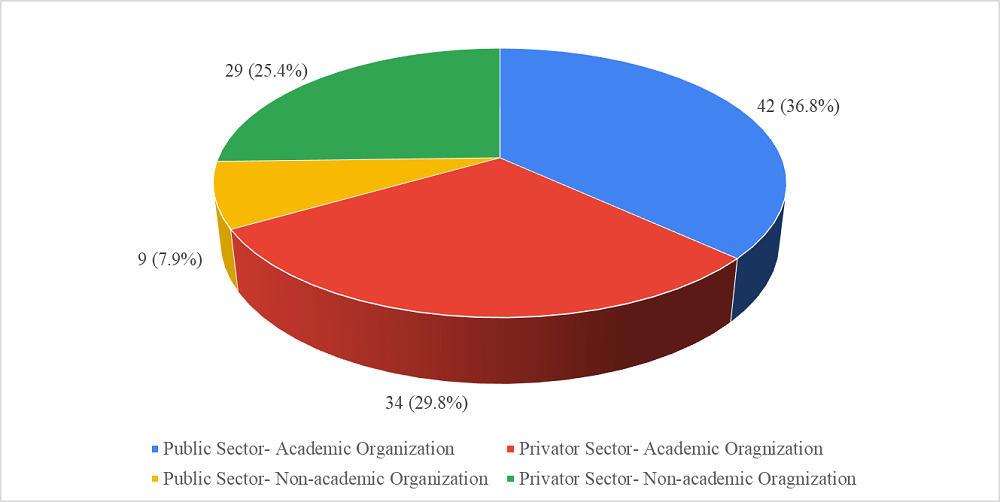
A total of 114 neonatologists/paediatricians involved in neonatal care and using surfactant in their clinical practice responded to the survey. The age of participants ranged from 28 to 68 years, median [interquartile range (IQR)] age was 38 (14) years, and mean (SD) age was 40.91±9.62 years. Among 114, 80(70.2%) were males and 34(29.8%) were females. Additional training in neonatology had been received by 39(34.2%) participants. All participants had five or more years’ experience of clinical practice in neonatal care, ranging from 5 to 42 years, median (IQR) duration of clinical experience was 9.50 (11) years, and mean (SD) was 11.50±9.23 years. There was almost equal representation of public and private sectors among the survey respondents. Seventy-six (66.7%) participants represented academic organisations and 38(33.3%) represented non-academic organisations (Figure 1).
Figure 1: Representation of Public and Private Sector among Survey Respondents
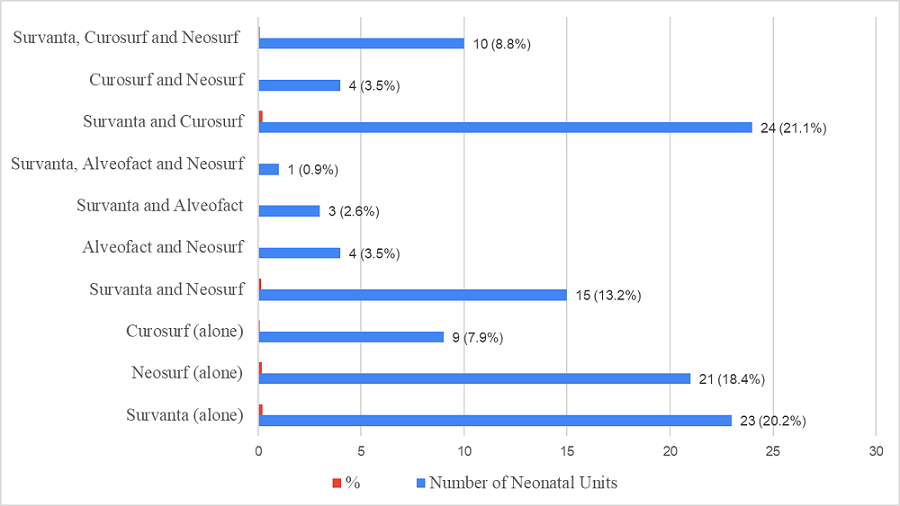
When asked about the preferred technique of surfactant administration, 69(60.5%) chose the InSurE (Intubate-Surfactant-Extubate) technique, 31(27.2%) replied that their choice depended on the clinical scenario, and 14(12.3%) mentioned LISA (Less Invasive Surfactant Administration) as their regular preferred choice. Although 61(53.5%) neonatal care units stocked two or more brands of surfactant in their inventory, the other 53(46.5%) stocked only one brand of surfactant. The usual options available in their pharmacy/inventory are shown in Figure 2. Only 38(33.3%) neonatal care units had both bovine and porcine surfactants available.
Figure 2: Surfactants available in surveyed Neonatal Units
When asked “Do you take parental consent before administration of surfactant?”, 104(91.2%) participants replied that they always do, 6(5.3%) replied they do it sometimes, and 4(3.5%) replied that they never take consent before administration of surfactant.
When asked “Do you inform parents about the animal origin of surfactant?”, 66(57.9%) participants replied that they never do, 36(31.6%) replied they do it sometimes, and 12(10.5%) replied that they always inform the parents about animal origin of surfactant.
Upon enquiry if the participants offer parents a choice in bovine or porcine source of surfactant, all 114(100%) replied with a “No”. The various reasons given by the participants for not giving the family a choice in bovine vs porcine origin of surfactant are summarised in Table 1.
Table 1: Justifications/reasons quoted by the respondents for not giving parents a choice between bovine and porcine surfactants
Sr. No. |
Reason |
N (%) |
1. |
I never thought over this subject | 3 (2.6%) |
2. |
I presume that it would be irrelevant for the parents | 73 (64.0%) |
3. |
I think that the family might be unable to decide | 7 (6.1%) |
4. |
I know that this issue is important but I am too busy to tell | 0 |
5. |
The choice of surfactant I offer to the patient is better than the others | 1 (0.9%) |
6. |
The choice of surfactant I offer to the patient is cheaper than others | 3 (2.6%) |
7. |
I want to offer the choice but I do not stock both bovine and porcine surfactants | 27 (23.7%) |
None of the variables (age, gender, special neonatology training, duration of clinical experience and place of work) was found to be associated with the practice of taking consent and informing parents about the animal origin of surfactant before its administration (Pearson Chi-square test- P ≥0.05 for all).
Discussion
Currently, there are four natural animal-derived surfactants available in the Indian market, ie, beractant (Survanta), poractant-alfa (Curosurf), Neosurf and bovactant (Alveofact). New synthetic surfactants with surfactant protein analogues contain no animal product and might be acceptable to strict vegans. They have also shown promise in clinical trials, but are not yet commercially produced [7, 8, 9]. The authors, with their clinical experience and communications with their colleagues, realised that paediatricians involved in neonatal care often face a dilemma whether parents should be informed about surfactant origin and whether neonatal units should stock more than one type of surfactant in order to allow parents make a choice.
Religious leaders do influence the opinions of people, but their opinions might vary even within the same religion, which is evident from the results of various surveys. A survey by Easterbrook et al [13], on the opinions of religious leaders in Australia regarding acceptability of porcine and bovine surgical implants in orthopaedic surgery, found that while the chairperson of a Hindu organisation did not accept the use of bovine surgical implants, Muslim and Jewish religious leaders permitted the use of porcine surgical products in dire situations. Another survey by Eriksson et al [14] surveyed religious and spiritual leaders of the six most prevalent religions worldwide, using a standardised questionnaire. Christians (including Jehovah’s Witnesses), Theravada Buddhists, and Jews had no objection to the use of drugs, dressings or implants with animal or human-derived contents. Jehovah’s Witnesses only objected to the use of blood derived products. The major branch of Hinduism, Vaishnavism, did not permit the use of any drugs, dressings or implants, containing porcine or bovine material, since they considered the killing of animals, especially of cows, as sinful. Sikhs also did not approve use of any animal-derived products. Sunni and Shia Muslims did not approve of drugs, dressings or implants with porcine content. However, in emergency situations, Hindus, Muslims and Sikhs, were all ready to waive these objections, if no other alternative drug existed and the treatment was considered life prolonging. All such decisions were left to the individual. A surgical team gathered the views of common religious groups in the United Kingdom (UK) [15]. They found that Islamic, Hindu, Sikh and Jain leaders had strong views on avoiding animal derived products, but the Christian and Jewish leaders did not. However, all religious leaders accepted the use of animal/human derived products if the procedure was performed to save life. In a survey of Hindu and Muslim religious scholars in the state of Gujarat [6], a Hindu religious scholar, and Trustee, of a leading institution of Gandhinagar, commented that although Hindus would not like to use a product of animal origin, they may be forced to do so when their well-being is at stake. A Muslim religious scholar of the Wakf Board, Government of Gujarat, opined that any medicine that cures disease may be utilised with informed consent.
Despite clear statements from religious leaders of all the major religions which, in general, clarify that animal-derived medications would be allowed in emergency situations, this does not mean that all adherents would have the same standpoint as the particular spiritual leader. Some parents may still not accept a view which permits the use of the prohibited animal product where there is no alternative. Neither can one conclude that the parents and family do not want to be informed about the origin of the surfactant.
A survey among 500 patients in a UK urology practice found that 43% patients would not like to use an animal-derived medication containing gelatine, even if no alternative were available [16]. Another study of 80 patients in an otorhinolaryngology out-patient clinic of a London hospital found that of all the patients including 25% vegetarians, 14% would ask their doctor whether their prescribed medicines contain animal-derived products, a small proportion (4%) would not even take the prescribed medicines which contained animal derived products. Interestingly, 70% vegetarians were vegetarians due to their lifestyle choice (70%) than to religious beliefs [17]. Sattar et al studied 100 patients at the Veterans Affairs Medical Center in Omaha, Nebraska [18], focussing on their perspective regarding pork or beef-derived medications. Out of 13 patients belonging to the faiths with dietary restrictions, 9(69.2%) were not aware that medications might contain beef and/or pork ingredients, 9(69.2%) replied that it was important to be informed before such a medication was prescribed, and 6(46.2%) would be willing to pay more for an alternative product that did not contain the prohibited ingredients.
It is on record that sometimes patients refuse such products even for life-saving treatments on religious grounds. Datz et al [5] described a young patient of the Islamic faith who declined the use of subcutaneous low molecular weight heparin for anticoagulation following a lower extremity orthopaedic procedure. In neonatology practice too, it has been frequently reported that families sometimes decline participation in surfactant trials on religious grounds. A Hindu family had wished to avoid the use of bovine surfactant, as cows are considered sacred in the Hindu religion, while a Muslim family avoided a porcine-derived surfactant [19].
Sherman et al [20] examined concerns regarding the use of animal derived medications among parents of neonates admitted to a neonatal intensive care unit in Wilmington, DE, USA. Of the 153 parents, 34% had concerns about animal-derived medications, 41% preferred a synthetic medication of equivalent efficacy, and 69% would like to be informed if a medication was animal-derived. The most important reason for parental concern was safety in using an animal product (49%), followed by religious beliefs (21%). Another study by Ahmed et al, surveying all the staff of a District General Hospital, NHS Trust, UK, and seeking their opinions as parents, received 151 responses. Approximately 11% preferred either bovine or porcine surfactants based on their religious beliefs, 36% preferred non-animal derived surfactant products and 53% had no preference. Seventy-four percent of the participants responded that the neonatal units should stock two types of surfactants, and 79% felt that the type of surfactant to be used for a preterm infant should be discussed in the antenatal period [21].
In the present survey, while most of the doctors (91%) reported always taking consent from parents/family before surfactant administration, only 10% informed them about the animal origin of the surfactant. None of them offered parents a choice in choosing a bovine or porcine sourced surfactant. It was surprising that a great majority (67%) of doctors had never thought over this subject or had presumed that it would be irrelevant for the parents. Quite a significant number (6%) even presumed that if they were to disclose this fact, the family might be unable to decide. Three participants believed that the choice of surfactant they offer to the patient is cheaper, which is not correct. In India, the maximum retail price of all natural surfactants per unit (mg) is the same ie, INR 77.9. One respondent even believed that the choice of surfactant (porcine derived) he/she offers to the patient is better than the other brands. However, it is the higher dose (200 mg/kg) of porcine surfactant which has shown a survival advantage when compared to 100 mg/kg of bovine surfactants. The head-to-head trials show similar efficacy among all surfactants used in similar doses [8].
The practices of Indian paediatricians/ neonatologists in the present study are similar to those across the world. Sattar et al [18] surveyed 106 physicians working at Veterans Affairs Medical Center in Omaha, Nebraska, USA for their knowledge and prescription practices for medications containing pork- and/or beef-derived ingredients. Although 70% thought it important to inform patients when such medications were prescribed, only 5(4%) reported actually doing so. In another large US survey of 2137 neonatologists, Bailey et al [22] found that only 2.2% neonatologists routinely discussed the animal origin of surfactant with parents. Access to only one surfactant was a major limitation. Another survey from England and Wales by Adappa et al [19] also discovered that only 9/42 (2.1%) doctors routinely discuss surfactant origin with the families. Only three units in England and the one in Wales stocked both porcine-derived and bovine-derived surfactants. The remaining units stocked only one or the other preparation.
The critical aspect of resource utilisation has also not been studied in India. In the present study, only 33% (38/114) of neonatal units in India were seen to stock both bovine and porcine preparations. Many respondents (27/114, 24%) mentioned that they want to offer the choice of surfactant to parents but they are not able to do so because they do not stock both bovine and porcine preparations. With many units choosing to stock only one surfactant, there are always concerns that informing parents about the animal origin of surfactant might result in a worse outcome for neonates. The attitudes and practices of our participants had no relationship to age, gender, any special training in neonatology, duration of clinical practice/experience, or place of work. The authors appreciate that the religious views of people in other countries cannot be extrapolated to the views of people in India and the lack of data on perceptions of Indian patients is a limitation. The surveys among prospective Indian parents to understand their perspectives regarding the use of animal origin surfactants may provide additional insights into this issue. However, results of any such surveys in one region cannot be generalised to others. People of different religions and faiths live in different countries, regions and socio-economic milieus which do affect their mindset. All major religions have subgroups with variations in their opinions. The religious beliefs of people change over generations and different opinions may exist even in the same family. Beside this, there is wide mixing of cultures in the present time. Notwithstanding this, the importance of keeping parents informed cannot be ignored. Parents need to be fully informed, not only of the importance of early administration but also of the nature of the available surfactants. We believe that when fully informed, most parents of today would agree to a life-saving medicine. However, this consent should not be presumed when there are reasons to believe it could be a potential problem, as also to dispel the perception that the medical profession has a dismissive attitude to patients’ beliefs. With the results of this survey, we hope to initiate discussion on this issue so that individual units can consider developing protocols on this subject with inputs from all stakeholders.
In preterm births, the family often receives counselling before the child is born. The antenatal period as well as this time may be taken as an opportunity to note their spiritual history and take relevant consents. Whenever required, the pharmacy departments should be involved in decision-making and choice of alternatives, if available. The regulatory bodies and licensing authorities should also ensure that manufacturers provide enough information on the product monographs with no ambiguity. Given the wide range of religions, castes and subcastes across our national population, healthcare providers should have the knowledge and understanding of backgrounds and beliefs of the local population. One should avoid making assumptions and being judgmental. Formal training programmes to equip caregivers with cultural competence should be in place to guide them on how to care for patients from various religious and cultural backgrounds.
Conflict of Interest: None to be declared
Funding: None to be declared
Statement of similar work: None to be declared
References
- Swihart DL, Yarrarapu SNS, Martin RL. Cultural Religious Competence in Clinical Practice. [Updated 2022 Nov 14]. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023 Jan [Cited 2023 Jul 2]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493216/
- Winiger F, Peng-Keller S. Religion and the World Health Organization: an evolving relationship. BMJ Glob Health. 2021 Apr; 6: e004073. https://doi.org/10.1136/bmjgh-2020-004073
- Polzer Casarez RL, Engebretson JC. Ethical issues of incorporating spiritual care into clinical practice. J Clin Nurs. 2012 Aug; 21(15-16): 2099-107. https://doi.org/10.1111/j.1365-2702.2012.04168.x
- Babos, MB, Perry JD, Reed SA, Bugariu S, Hill-Norby S, Allen MJ, et al. Animal-derived medications: Cultural considerations and available alternatives. J Osteopath Med. 2021 Mar 8; 121 (4): 361-370. https://doi.org/10.1515/jom-2020-0052
- Datz H, Syed A, Alsuhebani M, Tumin D, Tobias JD. Religious-related concerns and animal-derived medications during anesthetic care. Anaesth Pain Intensive Care. 2018 Apr-Jun [Cited 2023 Jul 2]; 22(2): 247-250. Available from: https://go.gale.com/ps/i.do?p=AONE&u=googlescholar&id=GALE%7CA553759883&v=2.1&it=r&asid=c5d3a1a6
- Goyal D, Goyal A, Brittberg M. Consideration of religious sentiments while selecting a biological product for knee arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2013; 21(7): 1577-1586. https://doi.org/10.1007/s00167-012-2292-z
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome – 2019 Update. Neonatology. 2019; 115(4): 432-450. https://doi.org/10.1159/000499361
- Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology. 2023; 120(1): 3-23. https://doi.org/10.1159/000528914
- More K, Nangia S, Ramaswamy VV, Sahni M. Surfactant Replacement Therapy in Neonates. Clinical Practice Guidelines. National Neonatology Forum, India; [Cited 2023 July 2]. Available from: https://nnfi.org/assests/upload/usefull-links-pdf/Surfactant_replacement_therapy_in_neonates-NNFI_CPG_Dec2021.pdf
- Ehman CJ. Religious Diversity: Practical Points for Health Care Providers. The University of Pennsylvania Health System; [Cited 2023 July 2]. Available from: https://www.uphs.upenn.edu/pastoral/resed/diversity_points.html
- Harrison RG. Religious And Cultural Beliefs- Guidelines. Blackpool, Fylde and Wyre Hospitals. NHS Foundation Trust; [Cited 2023 July 2]. Available from: https://www.bfwh.nhs.uk/wp-content/uploads/2015/07/Religious-And-Cultural-Beliefs.pdf
- Rumun AJ. Influence of Religious Beliefs on healthcare Practice. International Journal of Education and Research. 2014; 4: 37-48. ISSN: 2201-6333 (Print) ISSN: 2201-6740 (Online)
- Easterbrook C, Maddern G. Porcine and bovine surgical products: Jewish, Muslim, and Hindu perspectives. Arch Surg. 2008; 143(4): 366-70. https://doi.org/10.1001/archsurg.143.4.366
- Eriksson A, Burcharth J, Rosenberg J. Animal derived products may conflict with religious patients’ beliefs. BMC Med Ethics. 2013; 14: 48. https://doi.org/10.1186/1472-6939-14-48
- Koshy RM, Kane EG, Grocock C. A review of the use of biological mesh products in modern UK surgical practice: a religious and cultural perspective. Ann R Coll Surg Engl 2020; 102(8): 566-570. https://doi.org/10.1308/rcsann.2020.0114
- Vissamsetti B, Payne M, Payne S. Inadvertent prescription of gelatin-containing oral medication: its acceptability to patients. Postgrad Med J 2012; 88(1043): 499-502. https://doi.org/10.1136/postgradmedj-2011-130306
- Haarer F, Fu B, Desai K. Patients’ views on the availability of vegetarian medication in otorhinolaryngology: Our experience in an eighty patient questionnaire study. Clinical Otolaryngology. 2015; 40(4), 378–381. https://doi.org/10.1111/coa.12377
- Sattar SP, Ahmed MS, Madison J, Olsen DR, Bhatia SC, Ellahi S, et al. Patient and physician attitudes to using medications with religiously forbidden ingredients. Ann Pharmacother 2004; 38(11): 1830-1835. https://doi.org/10.1345/aph.1E0
- Adappa R, Benson R, Oddie S, Wyllie J. Use of animal surfactant: should we seek consent? Arch Dis Child Fetal Neonatal Ed. 2003; 88(4): F351. https://doi.org/10.1136/fn.88.4.F351-a
- Sherman TI, Moya F, Simmons PD, Kurtz D, Shaffer TH. Parental preferences regarding administration of an animal-derived versus a synthetic medication to newborn infants. J Neonatal Perinatal Med. 2016; 9(1): 7-14. https://doi.org/10.3233/NPM-16915062
- Ahmed SA, Arasu A. Another ethical dilemma in Neonatology. Archives of Disease in Childhood. 2011; 96: A72. https://doi.org/10.1136/archdischild-2012-302724.1050
- Bailey SM, Hendricks-Muñoz KD, Mally PV. Animal Origins of Surfactant: A Survey of Neonatologists’ Perceptions and Practices Regarding Parent Information Sharing. AJOB Primary Research. 2011; 2(1): 26-33. https://doi.org/10.1080/21507716.2011.566597