RESEARCH ARTICLE
Patients’ preferences on breaking bad news: a cross-sectional study from Iran
Kourosh Amini, Sahar Meshkini, Farhad Ramezanibadr
Published online first on June 17, 2023. DOI:10.20529/IJME.2023.039Abstract
Background: The sensitivity and skill of care providers, especially physicians, while communicating bad news to patients can improve patients’ acceptance of treatment and their emotional adjustment. We aimed to determine how to break bad news to cancer patients and consider their preferences in this regard.
Methods: This is a cross-sectional study in which 249 patients participated. The Poisson sampling method was used. Data were collected using the Measure of Patient Preferences (MPP) and patient demographic profile forms.
Results: Of the 249 participants, 178 (71.5%) were aware of their cancer diagnosis and 201 (80.7%) preferred to be informed of their cancer diagnosis. Patients’ preferences included: “Having his/her doctor take the time to answer all of his/her questions completely”, “Feeling confident about his/her doctor’s technical competence and skill”, and “His/her doctor telling him/her the best treatment option”.
Conclusion: According to our results, care providers should consider patients’ preferences in communicating and delivering bad news. Achieving this goal requires managers to plan for improving the communication skills of healthcare providers.
Keywords: breaking bad news, cancer, patient preference, bad news
Introduction
Despite the advances in treating and controlling cancer, it is still a deadly disease in many countries. Cancer patients usually encounter a high level of fear and anxiety for various reasons, such as the complexities of the treatments [1], recurrence, end-of-life care, burden to others, complications of treatment such as hair loss, impaired sexual function, disorders of various organs [2], cancer-related pain [3], etc. Due to this fear and anxiety among patients, we face several challenges when breaking bad news (BBN) in oncology units.
In 1984, Buckman first defined bad news as “… any information likely to alter drastically a patient’s view of his or her future (whether at the time of diagnosis or when facing the failure of curative intention)” [4]: p 1597]. BBN is regarded as a challenge to one’s communication skills. In other words, healthcare providers should develop special communication skills in breaking bad news, recognising patients’ communication needs, and considering situations [5]. It has been shown that BBN if presented in the right way, is necessary and vital in reducing the traumatic effects of bad news on patients’ illness perceptions [6], coping with disease [7], treatment adherence [8], and life expectancy [9], and may even encourage the patient to participate actively in complex decision making about treatment and disease [10].
Despite the importance of communication skills, healthcare systems worldwide do not emphasise their importance [11]. This neglect denies healthcare providers the ability and experience to deal competently with a patient in situations such as BBN [12]. As a result, they may avoid communicating with patients and thus ignore their communication needs [13] or fail to function sensitively in such a situation [4, 14].
A literature review shows that despite some clinical guidelines and strategies for breaking bad news to cancer patients, their preferences are not observed [15]. There is no specific guideline in Iran on breaking bad news to cancer patients [16]. Based on our search, only three such studies have been carried out in Iran [17, 18, 19]. Rozveh et al conducted one such narrative review study on cancer patients’ attitudes toward telling the truth and showed that members of the healthcare profession fall into two groups regarding telling the truth. One group feels telling the truth is stressful and harmful to patients. The other group considers awareness of the diagnosis to be the patients’ right and increases patients’ cooperation in the course of treatment. Moreover, the results of their study indicated that most patients wanted to know the true disease diagnosis. Finally, the authors concluded that the lack of knowledge among health professionals about how to break bad news, and ignoring patients’ preferences in delivering bad news were challenges for the profession [19]. In Germany, a study conducted by Goebel and Mehdorn demonstrated that all cancer patients prefer to receive enough information about the diagnosis and the treatment process through supportive communication according to their psychological needs. However, patients’ preferences are not observed in breaking bad news [20]. Additionally, some researchers and authorities have concluded that the willingness to receive bad news and deliver it to cancer patients depends on the cultural setting of patients [19]. Identifying patients’ preferences and defining related strategies and guidelines can help improve the delivery of bad news to cancer patients and promote patient outcomes. Therefore, the current study was carried out to determine how a cancer diagnosis is given and whether cancer patients’ preferences about delivering diagnoses are observed in Iran.
Methods
DesignThis was a cross-sectional study conducted from December 1, 2019 to March 1, 2020 in Iran.
SettingGenerally, when individuals in our community develop cancer symptoms, they tend to seek medical attention at non-governmental centres, especially private offices of general practitioners or internal medicine doctors. They do not usually prioritise visiting oncologists as their first choice for seeking medical care. If they are not financially stable, they go to government outpatient settings. If the doctors suspect cancer in these settings, they will refer the patient to an oncologist. If cancer is suspected, the oncologist will refer the patient to the hospital for further evaluation (such as determining tumour markers, biopsy, contrast-enhanced radiography, etc). Additionally, most patients are hospitalised for chemotherapy, although some may be outpatients based on their chemotherapy drug type. Finally, some patients are also hospitalised in oncology wards due to secondary cancer problems and/or side effects of treatments.
Given this context, the research environment included inpatient oncology ward #1 (with 32 beds and 19 nurses), inpatient oncology ward #2 (with 13 beds and four nurses), and inpatient internal medicine ward #1 (with 34 beds and 18 nurses) of Valiasr Hospital. At the time of the study, 21 specialists and 19 residents were providing medical treatment to cancer patients admitted to these three wards. A physician is usually responsible for one or more patients, and s/he does not intervene in the affairs of other patients except in emergencies. At the beginning of each shift, nurses in the ward are assigned two or three patients each and take care of them according to the ward routine or the doctor’s orders.
Sample and samplingInclusion criteria
(a) The inclusion criteria for participating patients were: being over 18-years-old and affected with cancer;
(b) not having a communication disorder, and
(c) not having a mental disorder — based on their doctor’s assessment;
(d) participating in the study voluntarily;
(e) getting a score higher than or equal to 70, according to the Karnofsky Performance Status Scale (KPSS) — a scale to measure the level of independence and performance of daily activities of the patient.
Exclusion criteria
(a) reluctance to participate in the study;
(b) psychiatric problems;
(c) age under 18;
(d) patients with communication disorders; and
(e) getting a score lower than 70, according to the KPSS
Sampling method
Since the main purpose of this study was to determine the proportion of patients whose preferences were met in presenting bad news, we used Formula 1 for the unknown population size [21] to calculate the minimum sample size.
Formula 1: n = (Z2 × [p × q]) ÷ d2
Based on this formula and with predetermined parameters: statistical power = 0.8, Z = value of α level of 0.025 in each tail = 1.96, p = the proportion of met needs = 70% = 0.7 (based on the most recent and closest study to our research at the time of preparing the study proposal [20]) q = (1 – p) = 0.30, d = acceptable margin of error = 0.07. Using the above values in Formula 1, the minimum number of patients required to complete the main study instrument or Measure of Patient Preferences (MPP) was determined to be 165 people.
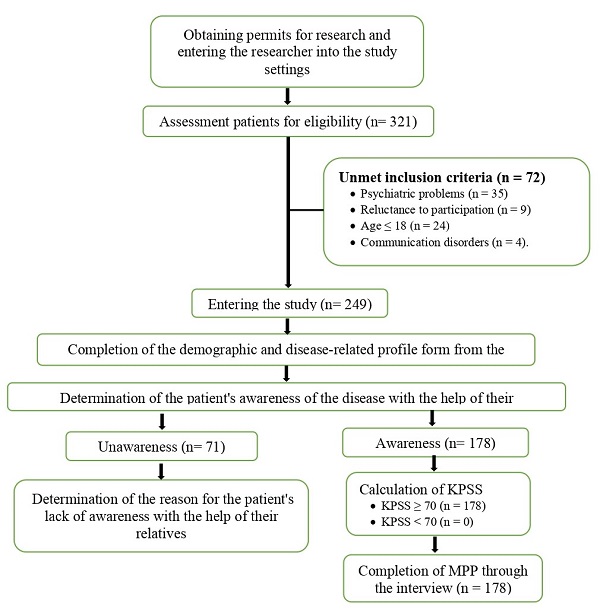
We used the Poisson distribution sampling method since the research population was unknown, and it was impossible to prepare a sampling framework [22]. In this way, we first numbered the three months that we considered for data collection in the research proposal as 12 weeks. Then, we randomly selected four weeks for each ward considered for the study. Accordingly, for four weeks, sampling (in the morning, evening, and night throughout the week) was done in a convenience sampling manner from each ward, while taking the inclusion criteria into account. During the data collection, 321 patients were evaluated for eligibility. Seventy-two patients were excluded due to the following: reluctance to participate in the study = 9; psychiatric problems =35 (depression = 11, anxiety and fear = 16, and substance use disorders = 8); age under 18 = 24; and communication disorders (aphasia = 3 and dysphonia = 1). Thus, 249 patients were included in the study, and the data about their demographic and disease-related characteristics were analysed.
Since filling out the primary tool of the study/MPP requires the patient to be aware of their illness, out of the 249 patients fulfilling the inclusion criteria, 71 patients who were not aware of their disease were not administered the questionnaire. Therefore, the data for 178 patients from the MPP questionnaires finally entered the statistical analysis stage.
It is worth noting that, apart from patients as the main participants in our study, 27 nurses and 12 doctors also participated. These care providers were selected through purposive sampling to answer questions about patients’ awareness or willingness to become aware of their disease and the causes of their unawareness.
Measures
Data collection tools included: (a) patient’s information and characteristics of the disease, (b) KPSS, and (c) MPP.
The patient’s information form had two parts. The first part contained questions regarding the demographic characteristics of patients and their disease-related details. The second part of this form consisted of three questions: (a) “Is the patient aware of the diagnosis of cancer?” (b) “Does the patient want to be aware of the diagnosis of cancer?”; (c) “What was the reason for the patient not being aware of the cancer diagnosis?” These questions were asked to physicians, nurses, and patients’ relatives.
KPSS is an important and standard tool in clinical practice [23], its scores ranging from 0 to 100. A higher score indicates better performance in the patient’s daily activities. Clinicians and researchers often use KPSS score ≥ 70 to enter patients into oncology protocols [24]. In other words, scores of 70 and higher indicate a patient’s independence in activities of daily life, and a lower than 70 score means dependence on others to perform activities of daily life [20, 25, 26, 27, 28]. So, we used KPSS score ≥ 70 as a criterion for patients to enter the study. The reliability and validity of the KPSS measures have been approved in several international studies and it has been used in many previous research studies around the world [20, 24, 25, 26, 27, 28]. Yaghmaie in 2006 translated this scale into Persian and recommended it for use in Iran and on Iranian cancer patients [29].
The MPP questionnaire was designed by Parker et al in 2001 [30], for examining the preferences of patients on how healthcare providers should break bad news to them. This questionnaire includes 46 items, and each item is scored according to its significance for the patient — scoring the questionnaires in the form of a 5-point Likert scale.
The items are scored as: 1 for “Essential, every doctor should do it”; 2 for “Very Important”; 3 for “Important”; 4 for “Optional, can take or leave it”; 5 for “Not at all important”.
To determine patients’ preferences, items that are scored ≤ 3 are considered patient communication preferences. Moreover, patients answer a yes/no format question concerning that item, as to “whether the physician had acted according to this preference”.
Although MPP has three subscales in its original version, studies have shown that these subscales have not been consistently replicated in cultural adaptations [31, 32, 33]. For this reason, in our study, we measured patient preferences generally based on Goebel and Mehdorn’s 2018 study [20].
To evaluate communication matches and mismatches, items’ scored ≤ 3 on the Likert scale were examined. We would achieve communication matches if the patient’s response to the question “whether the physician had acted according to this preference” was ‘yes’. If it was ‘no’, it would be considered as a communication mismatch. This was based on the method used by Goebel and Mehdorn [20]. Since patients might report any number of communication preferences, based on the method of Goebel and Mehdorn [20], we applied the proposed index for the description of whether patients’ preferences had been met (Formula 2). This index reflects the percentage of unmet communication needs and is called the Unmet Communication Needs Index (UCNI). Accordingly, a score higher than this index indicated that a significant number of communication preferences were not fulfilled.
Formula 2: UCNI = Number of reported communication mismatches ÷ Number of reported communication preferences × 100
We followed the forward-backward translation method since the MPP questionnaire was developed in English, and its Persian form was not psychometric. In this process, a translator familiar with the English language, whose mother tongue was Persian, translated the MPP into Persian. A meeting was held with the research team and an expert in the field of instrumentation, and the translation was approved. The Persian version was translated back into English by a second English translator, without seeing the original questionnaire. Another session was held with a composition similar to the first session. It was agreed to make changes after comparing the two main questionnaires and the translated one. The translated questionnaire in English was emailed to the instrument developer (Professor Parker) to confirm the correctness of the translated version, and her suggestions for some changes were incorporated. Another meeting was held with the first translator and the research team, and some changes were made to the Persian version. Finally, we distributed the prepared tool among ten cancer patients and obtained appropriate feedback regarding the comprehensibility and simplicity of the questionnaire items. To determine the reliability of this tool, we assessed its internal stability using Cronbach’s alpha coefficient (α = 0.884).
Procedure
During the three months of the study, the second author (SM) referred to cancer treatment centres — by observing the ethics of the research — and first filled in the patients’ demographic profile and disease characteristics form. Then, she inquired from the physicians or nurses of the patients about the awareness of the patient and their relatives regarding the disease diagnosis. The researcher also asked these professionals about the patient’s willingness to be made aware of the disease. Karnofsky’s functional index was calculated for them if the patient was aware of their illness. Thus, if they scored ≥70, by observing ethical considerations, the MPP was completed with a structured interview. A summary of the sample selection process and the completed questionnaires are shown in Figure 1.
Data analysis
We used descriptive and analytical statistical methods to analyse the data. Descriptive statistical methods — frequency or mean and standard deviation (SD) — were used to examine patients based on demographic variables and their awareness and willingness to know about the cancer diagnosis. The Chi-square test examined the relationship between the willingness to be aware and awareness of cancer diagnosis with demographic variables. Data analyses were performed in SPSS software version 16, and P ≤ 0.05 was regarded as significant.
Ethics approval
We obtained permission to use the MPP via email from the questionnaire designer. Before beginning the sampling, the study proposal was approved by the Biomedical Research Ethics Committee of Zanjan University of Medical Science (ZUMS), Zanjan, Iran (Ethics code: IR.ZUMS.REC.1398.239). Written informed consent was obtained from the patients and their first-degree relatives participating in the study. Participation in the study was voluntary. During the study, the principle of participants’ anonymity was observed. We were given permission to conduct the study by the Vice-Chancellor for Research and Technology of ZUMS to take the help of the officials, managers, physicians, and nurses working in the study setting.
Results
This study included 249 patients diagnosed with cancer from three wards of the hospital. The characteristics of the patients are shown in Table 1.
Table 1. Participants and disease-related characteristics
| Descriptive variables | Characteristics | N (%) |
| Gender | Male | 125(51.2) |
| Female | 124(49.8) | |
| Marital status | Single | 19(7.6) |
| Married | 195(78.3) | |
| Widowed | 35(14.1) | |
| Education | Illiterate | 97(39) |
| Elementary school | 86(34.5) | |
| High school diploma | 43(17.3) | |
| University degree | 23(9.2) | |
| Employment | Unemployed | 23(9.2) |
| Self-employed | 74(29.7) | |
| Employee | 45(18.1) | |
| Housewife | 98(39.4) | |
| Student | 2(.8) | |
| Worker | 7(2.8) | |
| Residence | Urban | 190(76.3) |
| Rural | 59(23.7) | |
| Family’s cancer history | Yes | 83(33.3) |
| No | 166(66.7) | |
| Cancer gradea | Grade I | 13(6.3) |
| Grade II | 86(42) | |
| Grade III | 88(42.9) | |
| Grade IV | 18(8.8) | |
| Relapse | Yes | 93(37.3) |
| No | 166(66.7) | |
| Treatment method Chemotherapy Radiotherapy Surgery |
Yes | 241(96.8) |
| No | 8(3.2) | |
| Yes | 45(18.1) | |
| No | 204(81.9) | |
| Yes | 97(39) | |
| No | 152(61) | |
| Cancer type | Gastrointestinal | 84(33.7) |
| Breast | 55(22.1) | |
| Haematological | 44(17.7) | |
| Reproductive & urinary | 24(9.6) | |
| Head & Neck | 15(6) | |
| Other types of cancer | 27(10.8) | |
| Mean ± SD | ||
| Age (Years) | 55.69 ±16.02 | |
| Duration since diagnosis (Months) | 15.45 ± 19.74 | |
| a Note: Out of 249 participants, the degree of illness was entered in the hospital records of 205. | ||
Awareness and wish to be aware of the disease diagnosis
Among the 249 participants, 201 (80.7%) wanted to be aware of their diagnosis, whereas 178 (71.5%) were actually aware of their diagnosis. Among 178 patients aware of their diagnosis, 49 (27.7%) received the cancer diagnosis from an internal medicine physician. Of the 178 patients, 105 (59%) received the news of cancer diagnosis in the doctor’s office. Among 71 patients who were not aware of their diagnosis, the decision not to inform the patient of the diagnosis in 53 (74.6%) cases was the preference of patients’ families. However, 43 (60.6%) of these 71 patients wanted to be informed about their disease (Table 2).
Table 2. Frequency based on awareness and willingness to be aware of the disease
| Variables | N (%) | |
| Aware of disease | Yes | 178(71.5) |
| No | 71(28.5) | |
| Sources of informationa | Haematologist and oncologist | 46(25.9) |
| Internal medicine specialist | 49(27.5) | |
| Surgical expert | 36(20.2) | |
| Gynaecologist | 15(8.4) | |
| Others | 32(18) | |
| Places of getting information | Doctor’s office | 105(59) |
| Inpatient department | 65(36.5) | |
| Emergency | 6(3.4) | |
| laboratory | 2(1.1) | |
| Reason for not being aware of the disease | Patient’s family preferences | 53(74.6) |
| Preferences of the patient’s physician | 18(25.4) | |
| Willingness to be aware of the disease | Yes | 201(80.7) |
| No | 48(19.3) | |
| Willingness to be aware of the disease among patients who were unaware | Yes | 43(60.6) |
| No | 28(39.4) | |
| a Note: Patients who are aware of disease (N = 178) | ||
Patients’ communication preferences
The study results on patients’ preferences showed that the patients who were aware of their disease diagnosis (n = 178) chose an average of 27.7 items out of 46 items in MPP as their communication preference. Table 3 shows the top 12 most essential patients’ communication preferences in frequency from high to low.
Table 3. Frequency of communication preferences reported by > 90% of patients and their rates of unmet communication needs index
| Item # | Patients’ communication preferences | N (%) | Mean (SD) | UCNI (%) |
| 16 | My doctor telling me the best treatment option | 173(97.74) | 4.76 (0.63) | 20 |
| 26 | Having my doctor take the time to answer all of my questions completely | 173(97.74) | 4.39 (0.83) | 47 |
| 24 | Feeling confident about my doctor’s technical competence and skill | 173(97.74) | 4.61 (0.78) | 17 |
| 28 | Being given enough time to ask all of my questions about my cancer and the available treatments | 171(96.61) | 4.13 (0.96) | 53 |
| 25 | My doctor being up to date on research on my type of cancer | 169(95.48) | 4.32(0.89) | 20 |
| 4 | The doctor setting enough time aside so he/she is not interrupted during our conversation | 167(94.36) | 4.12(1.03) | 32 |
| 12 | My doctor giving the information in clear, simple language | 166(93.78) | 4.53 (0.93) | 22 |
| 40 | My doctor telling me that he/she will do everything he/she can to cure my cancer | 166(93.78) | 4.55(0.98) | 25 |
| 5 | Having my doctor give me his/her full attention | 165(93.22) | 4.27(0.99) | 38 |
| 23 | My doctor telling me how my cancer may affect my daily functioning | 165(93.22) | 3.70(1.01) | 63 |
| 38 | Having my doctor really listen to me | 163(92.09) | 4.28(1.08) | 36 |
| 32 | The doctor telling me how I can get in touch with him/her to discuss additional questions and concerns before our next meeting | 161(90.96) | 4.23(1.17) | 75 |
| UCNI: Unmet Communication Needs Index | ||||
The unmet communication needs index was 48.46%. Items that had the highest non–compliance rate with patients’ communication preferences included:
1) The doctor gives me a written summary of the information to take home so that I can read it to help me remember the details of the conversation (item number: 46, UCNI = 98%);
2) Having the doctor tell me about resources in the community, for example, support groups (item number: 30, UCNI = 92.31%);
3) Having another healthcare provider (for example, a nurse) present to offer support and information (item number: 42, UCNI = 84.45%).
Relationship between demographic factors with awareness of diagnosis
The frequency of awareness regarding cancer diagnosis among participants in the middle-aged group (40-64 years) was significantly higher than both younger adults (18-40 years) and older adults (64+ years), as determined by X² = 39.81, p < 0.001. The frequency of participants with higher education who were aware of their diagnosis was higher than others (X² = 27.71, p <0.001). Housewives were more aware of the diagnosis (X² = 13.09, p = 0.033) than the other employment types. Most patients were unaware of their cancer diagnosis until the first chemotherapy session (X² = 20.00 P <0.001). In other words, the number of chemotherapy sessions was related to awareness of diagnosis. Higher-income group patients were more aware of their disease than those with lower income levels (X² = 10.45, p = 0.030). The frequency of disease awareness was higher among patients whose duration of illness was longer than those who had recently contracted the disease (X² = 21.47, P <0.001).
Other demographic and disease-related variables such as gender, marital status, residence, family history of cancer, grade of cancer, type of cancer, and cancer treatment methods had no significant relationship with awareness of cancer diagnosis.
Relationship between demographic factors and the willingness to be made aware of the diagnosis
Married people were also more willing than single people to be made aware of their disease (X² = 8.16, p = 0.015). Other demographic and disease-related variables were not significantly associated with the desire to be aware of the disease.
The study showed that the willingness to be informed about the cancer diagnosis is higher among middle-aged people than in other age groups (X2 = 17.59, p = 0.008).
Discussion
This study aimed to investigate BBN and cancer patients’ preferences in a city in north-western Iran. The results showed that most patients (71.5%) were aware of their diagnosis. Another study in Iran has confirmed this finding (73%) [17]. However, these numbers are contrary to the views of some medical professionals in Iran who believe that most cancer patients are not aware of their disease.
Regarding the rate of patients’ awareness of their disease, the results of studies vary in different countries. For example, Mansour et al in Saudi Arabia found that 86% of cancer patients were aware of their diagnosis [34]. In contrast, the degree of awareness of cancer diagnosis among patients in European and American countries is more than in the Middle East [35, 36, 37, 38]. However, Jie et al, in China, found that a little more than half of the patients (57%) were aware of their diagnosis [39]. In Iran and some Arab countries in the Persian Gulf and in Western countries, the practice of informing patients of a cancer diagnosis is more patient-centred. It emphasises patients’ autonomy and right to be informed of their disease. Whereas in China, disclosing the diagnosis is based on the opinions and preferences of relatives and patients’ families, who often want the doctor to conceal the diagnosis
In our study, we found most patients (80.7%) wanted to be aware of their diagnosis. In fact, this willingness was significantly stronger even among people who had not been made aware of their condition. This finding is compatible with other Iranian and foreign studies. For example, another Iranian study [18] has shown that 88.7% of patients in Tehran wanted to be made aware of their diagnosis. Similarly, studies conducted in Western countries, such as the investigation by Matsuyama et al in the United States [40], the survey by Wittmann et al in the United Kingdom [41], and studies in Asia, such as those by Mansour et al [34], and by Zekri et al in Saudi Arabia [42] are consistent with our research. They showed that most patients want to be aware of their diagnosis. Based on our literature review, only one study by Jie et al from China found that the percentage of patients who wished to be informed about their illness was significantly lower than in other studies and communities. However, overall it can be concluded that in most countries and regions, the majority of patients desire to be informed about their medical condition. Further research could reveal reasons for this discrepancy observed in the Chinese study [39].
Consistent with our results, the studies by Goebel and Mehdorn in 2018 [20] and Gebhardt et al in 2017 [43] in Germany showed that patients prefer to hear about their cancer diagnosis from a qualified person with sufficient knowledge of the disease.
Our study showed that 48.46% of patients’ preferences in receiving news about cancer diagnosis are not fulfilled. In other words, the non-compliance with patient preferences was high in our research population. In a review study by Kiesler and Auerbach, non-compliance with patients’ preferences was reported at 52% [44], which is almost identical to our findings. However, in studies in Germany by Goebel and Mehdorn [20] and Gebhardt et al [43], this rate is reported to be 30% and 25%, respectively, which is significantly less than that in our study. This inconsistency is probably due to cultural differences between the communities under investigation. On the other hand, paying too much attention to the treatment of cancer patients and neglecting their other needs and preferences could be another reason for this justification. This important issue needs to be considered more seriously by the healthcare providers in Iran.
The items that had the highest rate of non-compliance with patients’ preferences were presenting information in a written form and the need for a supportive communication. Goebel and Mehdorn [20] found that, in most cases, as in our study, the preference for written information from the healthcare providers were reported for non-compliance. It seems that the reason for healthcare providers not presenting written information is to prevent increased anxiety for the patient. In addition, providing such services is time-consuming, from the perspective of the healthcare providers, and time constraints and the high volume of work of health personnel prevent them from doing it. The study results of Goebel and Mehdorn [20] regarding supportive preferences were also consistent with our study. Likewise, they have stated that the high cost of cancer treatment should be mentioned as a factor in not attending to patients’ psychosocial needs, which is not considered cost effective, as shown in some previous studies [45, 46].
Based on demographic variables and disease characteristics, our study showed that: (a) a higher level of education is associated with increased awareness of a cancer diagnosis; (b) Awareness of cancer diagnosis is greater in middle-aged people; (c) Most patients were not informed of their cancer diagnosis until the first chemotherapy session. Patients’ delayed awareness of their disease diagnosis until the first chemotherapy can be attributed to a lack of respect for their right to know about a cancer diagnosis in Asian cultures. In Asian countries, including Iran, it is believed that telling the truth is stressful and harmful to the patient and should be withheld as long as possible [19, 47, 48]; (d) Housewives’ had greater awareness of their diagnosis, (e) as also people in a higher income bracket.
Limitations of the study
The most important limitation of our study lies in the fact that inaccessibility to accurate information about the stage of cancer in patients’ records eliminated the possibility of analysing the findings based on the stage of the disease. Second, since the breaking of news about the diagnosis is entirely dependent on the training received by healthcare providers, and patients’ preferences are based on their socio-cultural background, the generalisability of these results is subject to limitations. Third, this study examined the presenting of bad news only from the patients’ viewpoint and did not evaluate caregivers’ views.
Research recommendations
Considering the data of this study, hospital managers can try to improve the capabilities and skills of healthcare providers. In addition, implementing continuing education programmes on how to present bad news, can be helpful in improving the skills of those in this field, especially doctors. In this case, the healthcare providers can improve their performance by considering patients’ preferences and demands in communicating and breaking bad news and reducing physical and psychological complications in patients. It is recommended that the reasons for physicians’ avoidance of providing a written summary of information related to the diagnosis of cancer be investigated. This action will help to identify ways to facilitate it. Furthermore, it is recommended that physicians provide the necessary information about the resources available in the community, such as support groups, under the principles of therapeutic communication. It is also emphasised that doctors presenting bad news to patients should not be left to do it alone, and must be accompanied by another healthcare provider (for example, a nurse) for the patient to gain enough support and information. Due to the limitations of the present study, it is necessary to conduct more studies in other parts of Iran and the world, especially in Asia (Middle East) and the developing countries, to clarify this issue. The variable of disease stage should be considered an essential variable about the level of patients’ awareness or willingness to be made aware of the disease and their preferences. It is recommended that future studies examine and compare patients’ perspectives with those of care providers in this area, as well.
Conclusion
The current study indicated that most cancer patients want to be made aware of their diagnosis. Most of them are aware of their condition. They tend to receive complete information about the illness from skilled and professional people who consider their needs. However, in delivering news about a cancer diagnosis to the patients, their preferences are not observed.
Acknowledgement: This paper is part of an approved research project (ID: A-11-86-15), conducted with IR.ZUMS.REC.1398.239 code of ethics at Zanjan University of Medical Sciences. We are grateful to the research administration of Zanjan University of Medical Sciences, Zanjan, Iran, that financially supported the study. Moreover, we thank the staff of the cancer care centres of Zanjan city and cancer units of Valiasr teaching hospital and all participants of the study.
Funding: This work was supported by the Research and Technology Deputy of Zanjan University of Medical Sciences, Zanjan, Iran under Grant number: A-11-86-15.
References
- Wroot H, Afzal AR, Forbes C, Russell KB, Trepanier L, Patton M, et al. Fear of cancer recurrence among survivors of childhood cancer. Psychooncology. 2020;29(7):1132-40. https://doi.org/10.1002/pon.5387
- Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. https://doi.org/10.1136/bmj.k1415
- Sine H, Achbani A, Filali K. The effect of hypnosis on the intensity of pain and anxiety in cancer patients: a systematic review of controlled experimental trials. Cancer Investigation. 2021:1-19. https://doi.org/10.1080/07357907.2021.1998520
- Buckman R. Breaking bad news: why is it still so difficult? BMJ. 1984;288(6430):1597. https://doi.org/10.1136/bmj.288.6430.1597
- Winer E. Abstract ES7-1: Progression of disease and end of life: how to break bad news. AACR; 2020.
- Fraenkel L, McGraw S. Participation in medical decision making: The patients’ perspective. Med Decis Making. 2007;27(5):533-8. https://doi.org/10.1177/0272989X07306784
- Valizadeh L, Zamanzadeh V, Rahmani A, Howard F, Nikanfar AR, Ferguson C. Cancer disclosure: Experiences of Iranian cancer patients. Nurs Health Sci. 2012;14(2):250-6. https://doi.org/10.1111/j.1442-2018.2012.00686.x
- Schmitz FM, Schnabel KP, Bauer D, Woermann U, Guttormsen S. Learning how to break bad news from worked examples: Does the presentation format matter when hints are embedded? Results from randomised and blinded field trials. Patient Educ Couns. 2020;103(9):1850-5. https://doi.org/10.1016/j.pec.2020.03.022
- Pieters HC, Green E, Sleven M. “It Just Hit Me Like a Ton of Bricks”: Improving the patient experience of receiving a breast cancer diagnosis at an older age. Res Gerontol Nurs. 2021;14(2):79-89. https://doi.org/10.3928/19404921-20210115-02
- Brouwer MA, Maeckelberghe EL, van der Heide A, Hein IM, Verhagen EA. Breaking bad news: What parents would like you to know. Arch Dis Child. 2021;106(3):276-81. https://doi.org/10.1136/archdischild-2019-318398
- Back AL, Fromme EK, Meier DE. Training clinicians with communication skills needed to match medical treatments to patient values. J Am Geriatr Soc. 2019;67(S2):S435-S41. https://doi.org/10.1111/jgs.15709
- Biazar G, Delpasand K, Farzi F, Sedighinejad A, Mirmansouri A, Atrkarroushan Z. Breaking bad news: A valid concern among clinicians. Iran J Psychiatry. 2019;14(3):198.
- Moore PM, Rivera S, Bravo‐Soto GA, Olivares C, Lawrie TA. Communication skills training for healthcare professionals working with people who have cancer. Cochrane Database Syst Rev. 2018(7). https://doi.org/10.1002/14651858.CD003751.pub4
- Gan Y, Zheng L, Yu NX, Zhou G, Miao M, Lu Q. Why do oncologists hide the truth? Disclosure of cancer diagnoses to patients in China: A multisource assessment using mixed methods. Psychooncology. 2018;27(5):1457-63. https://doi.org/10.1002/pon.4545
- Bumb M, Keefe J, Miller L, Overcash J. Breaking bad news: An evidence-based review of communication models for oncology nurses. Clin J Oncol Nurs. 2017;21(5):573-80. https://doi.org/10.1188/17.CJON.573-580
- Janbabaei G, Hesamzadeh A, Esmaeili R. A review of approaches for disclosing cancer diagnosis to the patients. Clin Excell. 2014;3(1):12-28.
- Arbabi M, Rozdar A, Taher M, Shirzad M, Arjmand M, Ansari S, et al. Patients’ preference to hear cancer diagnosis. Iran J Psychiatry. 2014;9(1):8.
- Mollarahimi-Maleki F, Nojomi M, Rostami M-R. Attitude about cancer disclosure and quality of life of patients with cancer. Int J Med Health Res. 2016;5(7):457-66.
- Rozveh AK, Amjad RN, Rozveh JK, Rasouli D. Attitudes toward telling the truth to cancer patients in Iran: A review article. Int J Hematol Oncol Stem Cell Res. 2017;11(3):178.
- Goebel S, Mehdorn HM. Breaking bad news to patients with intracranial tumors: the patients’ perspective. World Neurosurg. 2018;118:e254-e62. https://doi.org/10.1016/j.wneu.2018.06.168
- Sim J, Saunders B, Waterfield J, Kingstone T. Can sample size in qualitative research be determined a priori? Int J Soc Res Methodol. 2018;21(5):619-34. https://doi.org/10.1080/13645579.2018.1454643
- Pfeffermann D, Rao CR. Sample surveys: design, methods and applications: Elsevier; 2009.
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. Evaluation of chemotherapeutic agents. 1949:191-205.
- Frappaz D, Taillandier L, Levard-Bonneville A, Sore J, Ricard D, Schiffler C, et al. P05. 28 Karnofsky performance score of brain tumor patients depends on clinician status. Neuro Oncol. 2018;20(Suppl 3):iii309. https://doi.org/10.1093/neuonc/noy139.354
- Chamberlain MC, Johnston SK, Glantz MJ. Neoplastic meningitis–related prognostic significance of the Karnofsky Performance Status. Arch Neurol. 2009;66(1):74-8. https://doi.org/10.1001/archneurol.2008.506
- Deng Z, Yu H, Wang N, Alafate W, Wang J, Wang T, et al. Impact of preoperative Karnofsky Performance Scale (KPS) and American Society of Anesthesiologists (ASA) scores on perioperative complications in patients with recurrent glioma undergoing repeated operation. J Neurorestoratology. 2019;7(3):3. https://doi.org/10.26599/JNR.2019.9040015
- Saarelainen LK, Turner JP, Shakib S, Singhal N, Hogan-Doran J, Prowse R, et al. Potentially inappropriate medication use in older people with cancer: Prevalence and correlates. J Geriatr Oncol. 2014;5(4):439-46. https://doi.org/10.1016/j.jgo.2014.07.001
- Péus D, Newcomb N, Hofer S. Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Medical Inform Decis Mak. 2013;13(1):72. https://doi.org/10.1186/1472-6947-13-72
- Yaghmaie F. Introducing a new scale for activities of daily living, Shaheed Beheshti Univ. Med Sci Health Serv-Fac Nurs Midwifery Q. 2006;15(50):3-12.
- Parker PA, Baile WF, de Moor C, Lenzi R, Kudelka AP, Cohen L. Breaking bad news about cancer: patients’ preferences for communication. J Clin Oncol. 2001;19(7):2049-56. https://doi.org/10.1200/JCO.2001.19.7.2049
- Eng TC, Omar K, Jaffar A, Shah SA, Yaakup H. Communication of bad news to cancer patients in a malay speaking country of south-east Asia: Validation of the Malay. Int Medical J. 2013;20(2):124-8.
- Fujimori M, Akechi T, Morita T, Inagaki M, Akizuki N, Sakano Y, et al. Preferences of cancer patients regarding the disclosure of bad news. Psychooncology. 2007;16(6):573-81. https://doi.org/10.1093/jjco/hyn159
- Mauri E, Vegni E, Lozza E, Parker PA, Moja EA. An exploratory study on the Italian patients’ preferences regarding how they would like to be told about their cancer. Support Care Cancer. 2009;17(12):1523-30. https://doi.org/10.1007/s00520-009-0621-7
- Mansour EA, Pandaan IN, Gemeay EM, Al-Zayd AH, Alenize EK. Disclosure of cancer diagnosis to the patient: A cross-sectional assessment of public point-of-view in Saudi Arabia. Arch Nurs Pract Care. 2017;3(2):038-44. https://doi.org/10.17352/anpc.000023
- Baile WF, Lenzi R, Parker PA, Buckman R, Cohen L. Oncologists’ attitudes toward and practices in giving bad news: an exploratory study. J Clin Oncol. 2002;20(8):2189-96. https://doi.org/10.1200/JCO.2002.08.004
- Costantini M, Morasso G, Montella M, Borgia P, Cecioni R, Beccaro M, et al. Diagnosis and prognosis disclosure among cancer patients. Results from an Italian mortality follow-back survey. Ann Oncol. 2006;17(5):853-9. https://doi.org/10.1093/annonc/mdl028
- Marwit SJ, Datson SL. Disclosure preferences about terminal illness: an examination of decision-related factors. Death Stud. 2002;26(1):1-20. https://doi.org/10.1080/07481180210144
- Sapir R, Catane R, Kaufman B, Isacson R, Segal A, Wein S, et al. Cancer patient expectations of and communication with oncologists and oncology nurses: the experience of an integrated oncology and palliative care service. Support Care Cancer. 2000;8(6):458-63. https://doi.org/10.1007/s005200000163
- Jie B, Qiu Y, Feng ZZ, Zhu SN. Impact of disclosure of diagnosis and patient autonomy on quality of life and illness perceptions in Chinese patients with liver cancer. Psychooncology. 2016;25(8):927-32. https://doi.org/10.1002/pon.4036
- Matsuyama RK, Kuhn LA, Molisani A, Wilson-Genderson MC. Cancer patients’ information needs the first nine months after diagnosis. Patient Educ Couns. 2013;90(1):96-102. https://doi.org/10.1016/j.pec.2012.09.009
- Wittmann E, Beaton C, Lewis W, Hopper A, Zamawi F, Jackson C, et al. Comparison of patients’ needs and doctors’ perceptions of information requirements related to a diagnosis of oesophageal or gastric cancer. Eur J Cancer Care. 2011;20(2):187-95. https://doi.org/10.1111/j.1365-2354.2009.01169.x
- Zekri J, Karim SM. Breaking cancer bad news to patients with cancer: A comprehensive perspective of patients, their relatives, and the public—example from a middle eastern country. J Glob Oncol. 2016;2(5):268-74. https://doi.org/10.1200/jgo.2015.001925
- Gebhardt C, Gorba C, Oechsle K, Vehling S, Koch U, Mehnert A. Breaking bad news to cancer patients: content, communication preferences and psychological distress. Psychother Psychosom Med Psychol. 2017;67(7):312-21. https://doi.org/10.1055/s-0043-113628
- Kiesler DJ, Auerbach SM. Optimal matches of patient preferences for information, decision-making and interpersonal behavior: evidence, models and interventions. Patient Educ Couns. 2006;61(3):319-41. https://doi.org/10.1016/j.pec.2005.08.002
- Brown V, Parker P, Furber L, Thomas A. Patient preferences for the delivery of bad news–the experience of a UK Cancer Centre. Eur J Cancer Care. 2011;20(1):56-61. https://doi.org/10.1111/j.1365-2354.2009.01156.x
- Richter D, Ernst J, Lehmann C, Koch U, Mehnert A, Friedrich M. Communication preferences in young, middle-aged, and elderly cancer patients. Oncol Res Treat. 2015;38(11):590-5. https://doi.org/10.1111/j.1365-2354.2009.01156.x
- Ehsani M, Taleghani F, Hematti S, Abazari P. Perceptions of patients, families, physicians and nurses regarding challenges in cancer disclosure: a descriptive qualitative study. Eur J Oncol Nurs. 2016;25:55-61. https://doi.org/10.1016/j.ejon.2016.09.003
- Liu Y, Yang J, Huo D, Fan H, Gao Y. Disclosure of cancer diagnosis in China: the incidence, patients’ situation, and different preferences between patients and their family members and related influence factors. Cancer Manag Res. 2018;10:2173. https://doi.org/10.2147/CMAR.S166437