CLINICAL TRIALS WATCH
DOI: https://doi.org/10.20529/IJME.2012.024
This issue of the Clinical Trials Watch provides data exclusively on trials that were registered in the Clinical Trials Registry of India (CTRI) during the year 2010. The factsheet was prepared with a manually created database using data retrieved from the CTRI website. However, as cautioned in the previous issue of Clinical Trials Watch, the data is subject to the dynamic nature of the CTRI website, which allows users to add, edit and remove records.
The data was collected from October to November, 2011, using search functions available in the CTRI website and identifying the trials that were registered in CTRI during 2010.
A new development in this issue’s factsheet is the introduction of classification of cities in which the trials are being conducted. The classification scheme used here is a modified version of the system developed by the Government of India (Ministry of Finance) for the ‘classification of cities/towns on the basis of 2001 Census’ (No.2(21)/E.II.(B)/2004). In the modified classification scheme used for this factsheet, we have only used one criterion- the House rent allowance parameter, to classify the cities. Also, we have considered the Class A and A-1 cities as ‘Class A’ which include metros and highly urbanised cities and Class B-1 and B-2 cities as ‘Class B’ which include medium sized cities and the Class C cities as ‘Class C’ which include smaller towns. Others which are not listed are counted as Unknown.
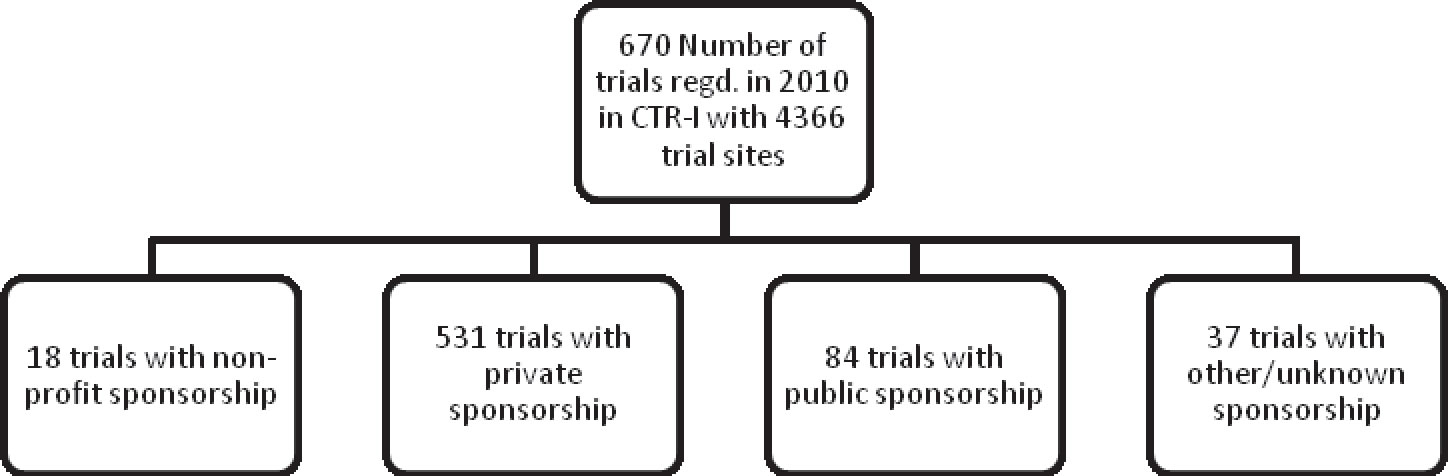
Among the 670 trials registered in CTRI in 2010, 88% of the trials were sponsored by private organizations, most of them being pharmaceutical companies (Table 1). This is indicative of the dominance of pharmaceutical companies on the clinical trials scene in India.
Phase III trials occupied the highest percentage (56%) of total trial settings registered in 2010. Among these trial settings, 67% were set in Class A cities (Table 2). These cities are highly urbanised and include all the metro cities in India.
Among these trial settings, a substantial proportion (94%) was privately funded trials (Table 3).
The data suggests a general trend of large number of trial settings in highly urbanized cities as compared to other cities in India. This may be due to the infrastructural facilities, higher patient population or ease of access to healthcare facilities that these cities are endowed with. This makes it preferable to conduct trials in these ‘Class A’ cities as compared to other areas. Other independent research conducted at the CSER indicates a migration of trial settings from Class A to Class B and Class C cities.
| Table 1. Trial status v/s Sponsor type | ||||
| Trial status | Sponsor type | |||
| Private | Non-Profit | Public | Unknown | |
| Not yet recruiting | 52 | 4 | 18 | 8 |
| Not applicable | 62 | 0 | 0 | 0 |
| Completed | 195 | 8 | 20 | 20 |
| Suspended | 8 | 0 | 0 | 0 |
| Open to recruitment | 203 | 6 | 46 | 9 |
| Other/terminated | 11 | 0 | 0 | 0 |
| Total | 531 | 18 | 84 | 37 |
| Table 2. City type v/s Trial Phase | |||||
| City type | Trial Phase | ||||
| Phase I | Phase II | Phase III | Phase IV | N/A | |
| Class A | 62 | 371 | 1645 | 641 | 162 |
| Class B | 12 | 68 | 437 | 167 | 36 |
| Class C | 6 | 27 | 118 | 28 | 12 |
| Unknown/Other | 8 | 52 | 251 | 219 | 44 |
| Total | 88 | 518 | 2451 | 1055 | 254 |
| Table 3. Sponsor type v/s City type | ||||
| Sponsor type | City type | |||
| Class A | Class B | Class C | Unknown | |
| Non-Profit | 32 | 12 | 14 | 1 |
| Private | 2688 | 577 | 277 | 559 |
| Public | 116 | 19 | 7 | 19 |
| Unknown | 30 | 2 | 3 | 10 |
| Total | 2866 | 610 | 301 | 589 |