COMMENTARY
Antimicrobial stewardship in low- and middle-income countries: Developing a broader perspective through an ethical analysis
Isha Sinha, Sonal Dayama
Published online first on November 22, 2023. DOI:10.20529/IJME.2023.072Abstract
The increase in the number of cases of antimicrobial resistance has gained attention worldwide. The main drivers of this situation are the misuse and overuse of antimicrobials for human and animal health. The imbalance between ensuring appropriate use of antimicrobials and providing equal access in the community makes this an ethical issue. The antimicrobial stewardship programme was initiated in response to this global crisis. Its framework includes interventions targeting the optimisation of antimicrobials in hospitals. Various countries have adopted stewardship interventions, and many success stories have been published. However, the steering of this programme faces hurdles due to the complexity that surrounds decision making in antimicrobial prescription, and the challenges of health systems in lower and middle income countries like India. Addressing these issues will extend the reach of these programmes, increase their sustainability and promote health-related justice to the community.
Keywords: antimicrobial stewardship programme, lower and middle-income countries, stewardship committee, community health ethics, pluralistic health system
Introduction
Antimicrobials are a life-saving medicine against many deadly infections faced by humankind. Not only do they treat individuals, they also provide shared benefits to the community by interrupting disease transmission. Hence, people who are not taking antimicrobials also benefit from their appropriate use.
Antimicrobial resistance (AMR) is a natural phenomenon that occurs due to microbial responses to these drugs. This is a significant public health concern because of the increasing burden of AMR, and the paucity of drugs in the research pipeline for newer antimicrobials [1]. Antimicrobials are the only class of drug whose population-level efficacy is reduced with excessive and inappropriate use over time [2]. It is estimated that by 2050, drug-resistant infections will lead to 10 million deaths annually, resulting in an overall loss of US $100 trillion [3]. This increases society’s moral responsibility to safeguard this precious good and prevent its misuse, looking ahead to the post-antimicrobial era, when it is assumed that there will be no new antimicrobials and the existing ones will no longer be effective against common infections [4].
Health inequities, resource constraints, and the unregulated exploitation of antimicrobials in lower and middle income countries (LMICs) are among the reasons for the increasing rate of AMR in such settings. It has been proven that per capita antimicrobial consumption is directly proportional to rates of AMR [5]. Globally, India ranks first in the per capita consumption of antimicrobials for human use, with a consumption rate of 10.7 units per individual in 2010. The increasing use of antimicrobials in agriculture and animal husbandry worsens the problem. To address this issue, the One Health concept proposes a collaborative approach with health authorities to curb the inappropriate antimicrobial usage in human health, animal health and the environment [6].
In 2015, the World Health Organization called for a Global Action Plan to combat this major public health crisis. An antimicrobial stewardship programme (AMSP) is one of the strategies described in the Plan intended to strike a balance between the benefits of early empirical drug therapy and the risks of inadvertently contributing to antimicrobials resistance. An AMSP aims to optimise antimicrobial use with safe and appropriate practices to improve clinical outcomes. This will indirectly reduce the cost of care and the harmful effects of overuse of antimicrobials [7]. An AMSP includes guidelines to ensure the appropriate choice of drug, its dosage, frequency, duration and route of administration based on diagnosis of the disease. In India, the Antibiotic Stewardship, Prevention of Infection and Control Programme was initiated by the Indian Council of Medical Research (ICMR) in 2012. The Programme aimed to bring faculty from multiple disciplines such as clinical pharmacology and microbiology to collaboratively plan strategies in response to the overwhelmingly increasing rate of AMR, recognising the need to implement feasible infection control practices at the hospital level [8]. A steering committee was formed in 2013 to guide AMSP activities in the country. A survey was conducted by the ICMR across 30 healthcare institutions to assess the existing stewardship activities in the country. It was found that 40% of institutions surveyed reported having an AMSP in place while 50% had set up multidisciplinary teams for this purpose, though not necessarily a designated AMSP committee. Hospital infection control practices were being implemented, infection control guidelines were in place, and AMR surveillance systems and prescription guidelines existed in these hospitals. However, implementation strategies such as formulary restrictions, preauthorisation guidelines, and feedback to prescribers were implemented by only 30% of healthcare institutions [9]. One reason could be the absence of the organisational structure required for the smooth functioning of the above-mentioned interventions. A systematic review identified similar problems in the lack of infrastructure; it also found poor surveillance and monitoring systems to be impeding AMSP activities. Infection control practices were the most commonly adopted measures in the context of AMSP activities. The study also revealed that a combination strategy was more beneficial than using a single intervention [10]. Such limitations in AMSP initiatives come in the way of an effective solution of the problem.
This article highlights ethical issues within AMSP activities and the existing challenges of the health system that impede the functioning of these programmes in LMICs like India. Addressing these issues will make AMSPs more efficient in reducing the burden of AMR that has ethical implications for community health. This article also emphasises the need for a broader engagement of the varied prescribers in LMICs to make programmes more effective with an extensive reach. AMSPs are a potential platform to address the inequities and increasing disease burden pertaining to antimicrobial use.
Ethical issues in current AMSP implementation in India
Lacunae in the stewardship committee
The stewardship committee is a multidisciplinary committee that implements AMSP activities on behalf of the health facility. The committee’s main functions are to implement AMSP activities, including regular antimicrobial auditing in selected departments, and monitoring antimicrobial susceptibility and resistance rates for a range of key indicator bacteria [11].
The committee’s composition varies in different settings but mainly comprises an infectious disease physician, infection control staff, a clinical pharmacist, a clinical microbiologist, an informatics specialist, a hospital epidemiologist, and a hospital administrator [1]. If these resources are not available, the facility may rely on “stewardship champions”. These can be physicians, nurses or pharmacists interested in AMSP [12].
However, most AMSP committees in resource-limited settings are led by doctors, considering their importance as prescribers, and rarely involve a pharmacist or a nurse despite their potential role in this area [13]. This creates a situation wherein a clinician’s choice of drug has to undergo scrutiny by another clinician. This may not be taken well by fellow prescribers, jeopardising the ultimate goal of antimicrobial optimisation. Hence, it is important to have members from different disciplines to minimise the conflict and add value to the decision making process. The inclusion of specialists like microbiologists, infectious disease physicians, nurses and pharmacists can help in the education and training of other staff, with a positive impact on AMSP activities[10]. Studies have shown that pharmacists and nurses are important stakeholders as they are involved in changing the line of treatment, under supervision, playing a crucial role in antimicrobial de-escalation from a higher to a lower power drug, based on close monitoring and clinical improvement. Their expertise in drug dynamics and their updated knowledge of newer drugs can assist clinicians in decision-making; they can also act as gatekeepers of antimicrobial usage in healthcare facilities. However, their role in decision making is influenced by interprofessional barriers existing within the hierarchies of the organisation. Including them and clearly defining their roles in AMSP can help overcome existing barriers, reaping benefits for the programme [14].
Patients’ perceived needs and the prescriber’s dilemma
A qualitative study conducted in Vellore found that patients had limited awareness of infectious diseases, antimicrobials and antimicrobials resistance. Health providers often have poor communication skills, further widening this gap, and adversely affecting the individual’s ability to participate in informed decision making. Due to their poor health literacy, patients tend to look for immediate symptomatic relief rather than wait for evidence-based treatment that can take time. Doctors find that the majority of patients who are prescribed a laboratory investigation do not come back for a follow-up visit, seeking the services of unauthorised pharmacies or healthcare providers instead.
The perception of patients that antimicrobials are the surest and fastest way of curing any ailment was one of the major drivers of antimicrobial prescription. Such practices accelerate drug resistance in the long run. They also violate the basic ethical principles of beneficence and non-maleficence. In the same study, pharmacists emphasised that increasing over-the-counter demand within a competitive and incentive-driven drug market has also led to increased antimicrobial prescription and usage [15].
The practice of self medication and the increasing demands for irrational prescription have changed the course of medicine sales over time. Increasing community awareness and reducing the patient-provider information gap by using information, education and communication materials will help public understand that antimicrobials also have limitations. Training and workshops are needed for practising doctors on upgraded guidelines with a strong emphasis on the importance of evidence-based antimicrobial usage AMR and its consequences. Inclusion of this subject in the medical curriculum will prepare future stewards in reducing the AMR burden. Laws ensuring that only authorised practitioners issue prescriptions, and mandating that pharmacies dispense antimicrobials only on prescription, must be implemented; prescription incentives by large pharmaceutical companies must be stopped. This will help in the judicious use of antimicrobials and regulate the drug flow within the market. Finally, there must be a reduction in the exorbitant prices of essential antimicrobials, in order to make essential medicines affordable and accessible to those who need them.
Lack of public participation in AMSP
Over the past few decades, patients have become recognised as important stakeholders in healthcare for improving health outcomes and reducing the cost of care [16]. However, AMSP interventions do not involve patients at any of the steps.
Two core interventions of AMSPs — formulary restrictions and prospective audit, and feedback to the prescriber — provide scope for exploring the role of patient participation. When crucial decisions on antimicrobial prescription are made without their involvement, patients get frustrated and may have anxieties and doubts regarding their disease condition. Such practices also raise ethical questions about patients’ right to information. Doctors and patients do not communicate their shared goals, though such communication is needed for correct diagnosis and appropriate treatment [17]. Proper patient-prescriber communication can play a crucial role in educating patients on antimicrobial usage and the consequences of misuse. It has been reported that the delayed prescription of antimicrobials for suspected viral upper respiratory tract infections, after proper communication between patient and doctor, has led to a reduction in prescriptions [18].
Active public participation should be encouraged in antimicrobials advisory groups. This will help the public understand the benefits of such interventions and their potential role in creating mass awareness [16]. The involvement of local “antimicrobials champions” as public advocates can create awareness of rational antimicrobial usage, the threat of resistant infections, the measures to be taken while dealing with sick patients, and the need to abstain from self medication. Patient groups can act as a bridge between the community and healthcare facilities. Such approaches can reduce the patient-provider information gap and upgrade patients from being mere beneficiaries to becoming partners. Efforts to engage patients and prescribers in a transparent and meaningful way, with the objective of patient welfare, can help foster trust with the potential for a successful AMSP [19].
Strengthening the stewardship committee, implementing measures to minimise the prescriber’s dilemma, and promoting community participation at different levels will increase the effectiveness of AMSP. This will lead to outcomes that are just and more sustainable.
Challenges of the existing health system
The AMSP framework is challenging to incorporate within overburdened and understaffed health systems. Most studies reporting the success of AMSP interventions are from high income countries with well organised health sectors, strong drug regulation, and a lower burden of infectious diseases [20]. This is in contrast to the situations prevailing in LMICs. A high infectious disease burden, lack of political commitment, poor funding of the health sector, shortage of skilled manpower, inadequate laboratory facilities, frequent stockouts of essential antimicrobials in public health facilities, and a weak surveillance system — all these pose challenges in the implementation of AMSP. This is further complicated by inappropriate drug prescribing practices, prescriptions by unregistered providers, over-the-counter drug dispensing, poor health literacy, the overuse of expensive reserve antimicrobials that make treatment less affordable to many people in LMICs, and a profit-driven private drug market with incentives that create the impression that antimicrobials are essential for all illnesses [21].
Lax drug regulation in LMICs results in misuse and waste of this lifesaving product. With a privatised health system and the lack of access to basic primary healthcare, antimicrobials are available to those who can afford them while depriving the poor who are more vulnerable to infectious diseases due to their poverty, malnutrition, poor access to clean water and sanitation. Many researchers argue for addressing the social determinants of infection susceptibility and inequity within social hierarchies to reduce the burden on AMSP [19].
Furthermore, antimicrobial usage in LMICs is like a double-edged sword. On one hand, the lack of drugs results in higher infectious disease mortality and on the other, overuse and misuse of drugs has resulted in higher rates of AMR. Hence, antimicrobial usage requires knowledge and understanding to reap its benefits and minimise its harms. For example, carbapenems are an expensive class of drugs that are the first-line of choice for community-acquired infection caused by Extended Spectrum Beta-Lactamase (ESBL) organisms. However, excessive use of carbapenems has resulted in an increasing prevalence of multi-drug-resistant gram-negative pathogens, contributing to morbidity and mortality in hospital intensive care units.
Many essential drugs such as carbapenems, higher generation cephalosporins, etc are expensive and unaffordable for the common public. Hence, cheaper antimicrobials that are old and toxic are used instead of an expensive alternative that is less toxic. Individuals are left with no option but to use these less expensive, and less effective, drugs. [22].
Thus, AMSPs should take up the dual responsibility of safeguarding antimicrobials and making efforts to overcome the inequities in availability of costly but effective antimicrobials for the treatment of infections. Stewardship means a responsible utilisation of antimicrobials, balancing the present-day need for appropriate treatment against future needs, for a sustained community benefit [23]. The plans of “saving for the future” will remain meaningless if the needs of today are compromised.
The urgent need for a wider engagement of AMSPs in LMICs
Although efforts have been made to customise AMSP based on the capacity and need of each setting, the importance of a people-centric approach needs more emphasis [7]. The limited focus of AMSP ignores a broader population that does not enter healthcare facilities but is vulnerable to resistant infections and has poor access to quality drugs. The following measures should be adopted to expand the AMSP framework in LMICs:
Strengthening local surveillance data
Most surveillance reports are from hospital data and not generalisable to the community [24]. A 2019 scoping review, including AMSP studies from LMICs, found that data were available in public urban health facilities with little or no data from rural areas and the private sector [12]. There is lack of robust microbiological surveillance data for the common organisms causing infection, antimicrobial susceptibility, and the community burden of multidrug-resistant infections, all needed to guide the development of rational antimicrobials protocols.
Engaging with pluralistic health systems in LMICs
In the context of drug dispensing practices in LMICs, the burden of AMR reported from healthcare facilities may be just the tip of the iceberg. The mixed/pluralistic health system in LMICs caters to a wider population that uses both government and private facilities, depending on their need, affordability, and ease of access [25]. These healthcare and drug providers vary in their training and skills. They include both licensed medical practitioners and unlicensed providers, with the latter catering to more than half of the people seeking outpatient care. Unlicensed providers contribute to the huge market of community drug retail outlets in LMICs that dispense drugs without the mandatory prescription. They function outside any regulatory framework. Surprisingly they find no space in AMSPs. Although drug regulatory laws in many countries prohibit dispensing of prescription-only drugs without a prescription, there is a poor adherence to this requirement. A systematic review in 2021 reported that between 50% and 93.8% of antimicrobials were dispensed without prescription, with a pooled prevalence of 78.8% (95% CI: 65-89%) in LMICs [26]. This is because of an unregulated market that is more responsive to consumer demands [27].
On the other hand, some studies have also explored the benefits of easy availability of drugs in reducing morbidity and mortality [20]. This creates a difficult choice: should drug regulations should be enforced strictly, further restricting their availability, or should one accept a nominally illegal practice that is also a mode of access to antimicrobials for those who cannot afford treatment in hospitals [20]? However, it is also important to remember that poor drug quality and inappropriate dosing provide a conducive environment for the microorganism to develop resistance. Easy availability of broad-spectrum antimicrobials with minimal side effects (severe side-effects would otherwise discourage overuse) have encouraged self-medication. This leads to exploitation of this non-renewable good over time. The major implications include misuse of antimicrobials, inequitable drug distribution, and diverting patients from the correct treatment. This has resulted in colonisation, in the community, of multi drug-resistant pathogens that were once thought to be limited to hospital premises. Even long-term facilities are becoming hot spots for such resistant organisms due to increasing antimicrobial usage.
Hence, the efforts of AMSPs to optimise antimicrobial use will remain futile without regularising the huge drug market existing outside healthcare facilities. It is imperative to have an inclusive approach and bring pluralistic health systems and private drug markets under the jurisdiction of AMSPs. Universal access to drugs should have a broader perspective focusing on the under-served who suffer due to unequal distribution and poor access to newer antimicrobials. It should also ensure the availability of effective antimicrobials for future generations.
The roles of different players
The participation of all stakeholders who are involved in drug prescription and drug consumption is crucial to achieve the vision of AMSP. At the facility level, leadership and commitment are required in establishing guidelines and implementing stewardship activities. A multidisciplinary stewardship committee can extend its services outside the hospital to improve the knowledge of various prescribers and the local community. In prescribing antimicrobials, doctors have responsibilities towards the individual patient as well as the community. It is important to engage with informal prescribers and identify their roles in communicating information, adherence to guidelines and creating a positive incentive that motivates them to adopt such practices [20]. Governments and health departments have the responsibility to put in place policy steps to conserve antimicrobials and strengthen existing laws for drug usage. The community should participate responsibly in informed decision making for correct treatment and refrain from self-medication.
Ethical approach for implementing AMSPs
Stewardship activities should be carried out with the aim of ensuring dignity of life and health equity. This entails responsible usage of existing antimicrobials (by ensuring appropriateness of drug choice, dose frequency and duration) and equitable access to the newer and more effective agents at a reasonable price. They should emphasise the principle of “universal access to healthcare” to reduce the distributive injustice of antimicrobials availability.. To meet their responsibility towards future generations, AMSPs must ensure availability, affordability and access to quality drugs. This will also reduce the burden of “difficult to treat” infections that require expensive antimicrobials.
Childress et al. have articulated a set of moral considerations. These are values, principles or rules relevant in the context of public health, and do not align with any particular theory but are similar to many approaches [28]. Addressing the challenges presented in this article will also ensure compliance with these moral considerations.
Figure 1 depicts the moral considerations that an AMSP can apply in its framework to enhance its functioning and address the issues pertaining to the antimicrobial crisis.
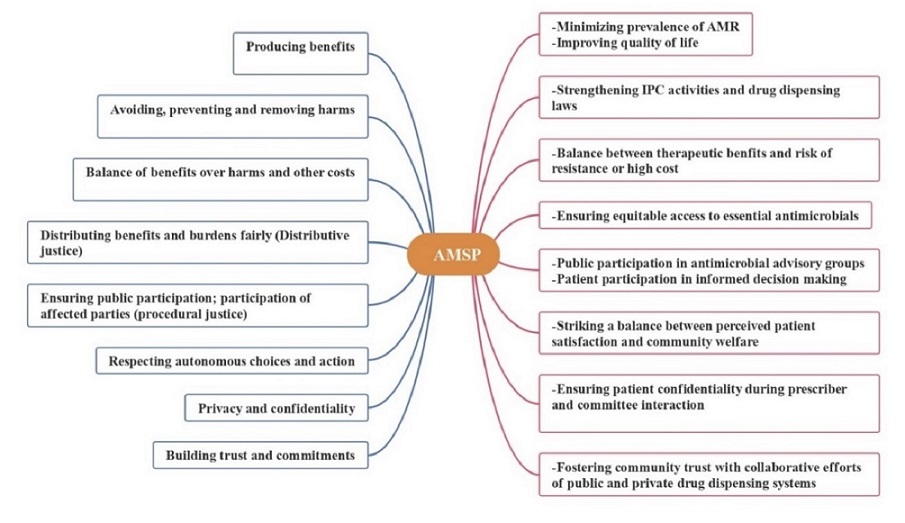
Figure 1: Scope of AMSP framework based on moral considerations for community health
Conclusion
The concept of an AMSP holds promise for the future. It can play a pivotal role in reducing distributive inequity and the infectious disease burden of the community in LMICs. It is important to understand that the multifactorial problem of AMR cannot be tackled solely by AMSP initiatives, and a more comprehensive and collaborative approach is required. Health systems strengthening, ensuring a robust surveillance and monitoring system, addressing the social determinants of health, addressing the challenges in implementation of AMSP, promoting medical education and training, public participation, and engaging with pluralistic health systems will all play a crucial role in ameliorating the antimicrobial crisis holistically, ensuring sustained benefits for the community in the long run.
Acknowledgement: The authors would like to acknowledge the team of Rural Women’s Social Education Centre (RUWSEC) & Thakur Foundation for the fellowship through this writeshop. We also express our gratitude to the mentors and fellow participants for their valuable feedback during the writeshop.
References
- MacDougall C, Polk, RE. Antimicrobial stewardship programs in health care systems, Clin Microbiol Rev. 2005 Oct;18(4): 638–656. https://doi.org/10.1128/cmr.18.4.638-656.
- Yen CF, Cutrell JB. Antimicrobial ethicists: Making ethics explicit in antimicrobial stewardship. Antimicrob Steward Healthc Epidemiol. 2021Jul 30;1(1): e17. https://doi.org/10.1017/ash.2021.181
- Ciapponi A, Bardach A, Sandoval M, Palermo M, Navarro E, Espinal C, et al. Systematic Review and Meta-analysis of Deaths Attributable to Antimicrobial Resistance, Latin America. Emerg Infect Dis. 2023;29(11): 2335-2344. https://doi.org/10.3201/eid2911.230753
- Hansson K, Brenthel, A. Imagining a post-antibiotic era: a cultural analysis of crisis and antibiotic resistance. Med Humanit. 2022 Sep;48(3): 381–388. https://doi.org/10.1136/medhum-2022-012409
- Farooqui HH, Selvaraj S, Mehta A, Heymann DL. Community level antibiotic utilization in India and its comparison vis-à-vis European countries: Evidence from pharmaceutical sales data,” PLoS One. 2018 Oct 17;13(10): e0204805. https://doi.org/10.1371/journal.pone.0204805
- Taneja N, Sharma M. Antimicrobial resistance in the environment: The Indian scenario. Indian J Med Res. 2019 Feb;149(2): 119–128. https://doi.org/10.4103/ijmr.ijmr_331_18
- Cox JA, Vlieghe E, Mendelson M, Wertheim H, Ndegwa L, Villegas MV,et al. Antibiotic stewardship in low- and middle-income countries: the same but different?, Clin Microbiol Infect. 2017 Nov; 23(11): 812–818. https://doi.org/10.1016/j.cmi.2017.07.010
- Chandy SJ, Michael JS, Veeraraghavan B, Abraham, OC, Bachhav SS, Kshirsagar NA. ICMR programme on Antibiotic Stewardship, Prevention of Infection & Control (ASPIC), Indian J Med Res. 2014 Feb;139(2): 226–230. PMID: 24718396.
- Walia K, Ohri VC, Madhumathi J, Ramasubramanian V. Policy document on antimicrobial stewardship practices in India. Indian J Med Res. 2019 Feb;149(2):180–184. https://doi.org/10.4103/ijmr.ijmr_147_18
- Sahni A, Bahl A, Martolia R, Jain S, Singh S. Implementation of antimicrobial stewardship activities in India. Indian J Med Spec. 2020;11(1):5. http://dx.doi.org/10.4103/INJMS.INJMS_118_19
- World Health Organization. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit. Geneva: WHO; 2019 Oct [Cited 2023 Oct 10]. Available from: https://www.who.int/publications/i/item/9789241515481
- Pierce J, et al., “Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries: A position statement for the international society for infectious diseases,” International J Infect. Diseases, 2020 Jul; 96: 621–629. https://doi.org/10.1016/j.ijid.2020.05.126
- Nampoothiri V, et al., “What does antimicrobial stewardship look like where you are? Global narratives from participants in a massive open online course,” JAC-Antimicrob Resist. 2022 Feb; 4 (1): dlab186, https://doi.org/10.1093/jacamr/dlab186
- Broom A, Plage S, Broom J, Kirby E, and Adams J, “A qualitative study of hospital pharmacists and antibiotic governance: negotiating interprofessional responsibilities, expertise and resource constraints,” BMC Health Serv. Res., 2016 Feb; 16 (1): 43. https://doi.org/10.1186/s12913-016-1290-0
- Chandy S, Mathai E, Thomas K, Faruqui A, Holloway K, and Lundborg C, “Antibiotic use and resistance: perceptions and ethical challenges among doctors, pharmacists and the public in Vellore, South India.,” in IJME FOURTH NATION. BIOET. CONFER. REP.
- Foy R et al., “Revitalising audit and feedback to improve patient care,” BMJ, 2020 Feb: 213. https://doi.org/10.1136/bmj.m213
- Parsonage B, Hagglund P, Keogh L, Wheelhouse N, Brown R, and Dancer S, “Control of Antimicrobial Resistance Requires an Ethical Approach,” Front. in Microb., 2017 [Cited on 2022 Jun 09; 8. Available from: https://www.frontiersin.org/article/10.3389/fmicb.2017.02124
- Drekonja D, et al., “Antimicrobial Stewardship in Outpatient Settings: A Systematic Review,” Infect. Cont. & Hosp. Epidemio., 2015 Feb; 36 (2): 142–152. https://doi.org/10.1017/ice.2014.41
- V. Gopichandran, “Reducing the Ethical Burdens of Antimicrobial Stewardship using a Social Determinants Approach,” Asian Bio. Rev., 2022;14 (2). https://doi.org/10.1007/s41649-022-00202-9
- Merrett G, Bloom G, Wilkinson A, and MacGregor H, “Towards the just and sustainable use of antibiotics,” J of Pharm Policy and Pract, 2016 Oct; 9 (1): 31. https://doi.org/10.1186/s40545-016-0083-5
- Gebretekle G et al., “Opportunities and barriers to implementing antibiotic stewardship in low and middle-income countries: Lessons from a mixed-methods study in a tertiary care hospital in Ethiopia,” PLoS One, 2018 Dec; 13 (12): e0208447. https://doi.org/10.1371/journal.pone.0208447
- Shlaes D and Bradford P, “Antibiotics—From There to Where?: How the antibiotic miracle is threatened by resistance and a broken market and what we can do about it,” Pathog Immun, 2018 Feb; 3 (1): 19–43. https://doi.org/10.20411/pai.v3i1.231
- Dyar O, Huttner B, Schouten J, Pulcini C, “What is antimicrobial stewardship?,” Clinical Microb. and Infect., 2017 Nov, 23, (11):793–798. https://doi./10.1016/j.cmi.2017.08.026
- “AMRSN_annual_report_2020.pdf.”. [Cited 2022 Jun 02]. Available from: https://main.icmr.nic.in/sites/default/files/guidelines/AMRSN_annual_report_2020.pdf
- Meessen B et al., “Composition of pluralistic health systems: how much can we learn from household surveys? An exploration in Cambodia,” Health Pol. and Plan., 2011 Jul; 26 (1): i30–i44. https://doi.org/10.1093/heapol/czr026
- Torres N, Chibi B, Kuupiel D, Solomon V, Mashamba-Thompson T, Middleton L, “The use of non-prescribed antibiotics; prevalence estimates in low-and-middle-income countries. A systematic review and meta-analysis,” Arch. of Pub. Health, 2021 Jan; 79 (1): 2. https://doi.org/10.1186/s13690-020-00517-9
- Belachew S, Hall L, Erku D, and Selvey L, “No prescription? No problem: drivers of non-prescribed sale of antibiotics among community drug retail outlets in low and middle income countries: a systematic review of qualitative studies,” BMC Pub. Health, 2021 Jun; 21(1):1056. https://doi.org/10.1186/s12889-021-11163-3
- Childress J et al., “Public Health Ethics: Mapping the Terrain,” J. Law. Med. Ethics, vol. 2002; 30 (2):170–178. https://doi.org/10.1111/j.1748-720X.2002.tb00384.x