THEME – ETHICAL AND LEGAL CHALLENGES OF VACCINES AND VACCINATION
Lessons learnt in Japan from adverse reactions to the HPV vaccine: a medical ethics perspective
Hirokuni Beppu, Masumi Minaguchi, Kiyoshi Uchide, Kunihiko Kumamoto, Masato Sekiguchi, Yukari Yaju
DOI: https://doi.org/10.20529/IJME.2017.021
Abstract
The human papillomavirus (HPV) vaccine has been linked to a number of serious adverse reactions. The range of symptoms is diverse and they develop in a multi-layered manner over an extended period of time. The argument for the safety and effectiveness of the HPV vaccine overlooks the following flaws: (i) no consideration is given to the genetic basis of autoimmune diseases, and arguments that do not take this into account cannot assure the safety of the vaccine; (ii) the immune evasion mechanisms of HPV, which require the HPV vaccine to maintain an extraordinarily high antibody level for a long period of time for it to be effective, are disregarded; and (iii) the limitations of effectiveness of the vaccine. We also discuss various issues that came up in the course of developing, promoting and distributing the vaccine, as well as the pitfalls encountered in monitoring adverse events and epidemiological verification.
Introduction
In this paper, we review the adverse reactions following human papilloma virus (HPV) vaccination in Japan, and the measures taken by the Ministry of Health, Labour and Welfare (MHLW) (1) to withdraw active recommendation of the vaccine. These measures triggered domestic and international controversy. We also discuss various problems that occurred while developing, promoting and distributing the vaccine; the pitfalls encountered in monitoring adverse events and epidemiological verification; and the influence of big pharma on healthcare policy and research.
- Overview of the HPV vaccine issue in Japan
- The problem with the HPV vaccine: refuting the GACVS statement (19)
- Safety issues
- Genetic basis of autoimmunity
- Coding and the loss of important information
- Paradigm shift
- Effectiveness
- Structural flaws: an ethics viewpoint
- Immunisation Act and HPV vaccine promotion by manufacturers
- Pressure from outside Japan
- Medical professionals forgetting their role
- Considerations for solving problems
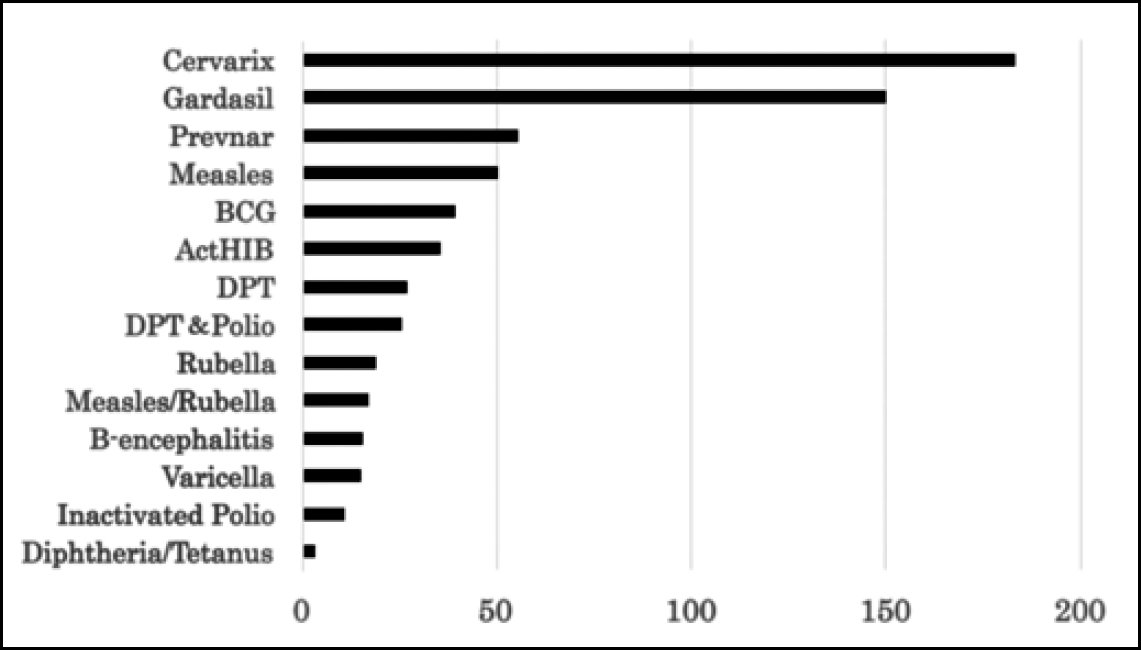
HPV vaccines were approved later in Japan than in the western countries (October 2009 for Cervarix, and July 2011 for Gardasil). The vaccination rate was initially low. However, after a campaign for the promotion of the vaccine, which led to government subsidisation of the cost of the vaccine in November 2010, the vaccination rate increased exponentially. This was followed by an unexpected increase in reports of adverse events (AEs). Importantly, these vaccines gave rise to a large number of serious AEs. Table 1 shows the number of reports of serious AEs/adverse drug reactions (ADRs), defined according to the ICH E2A guidelines (2), submitted with respect to HPV vaccines by vaccine manufacturers and medical professionals at the end of February 2016 (3). These numbers far exceed those for other vaccines, even if one allows for the probability that vigilance would be higher for a newly introduced vaccine than an older, time-tested one (4, 5) (Fig. 1). As these data have been compiled from voluntary reports, the actual incidence of AEs may well be far higher (6, 7).
| Table 1: Reports of serious AEs/ADRs of HPV vaccines in Japan (3) | ||||
| Vaccines | Total dose* | Total number of inoculated persons* | Serious AE/ADR reports | |
| From MAH | From medical institutes | |||
| Cervarix | 6,998,266 | 2,590,000 | 835 | 448 |
| Gardasil | 1,924,121 | 800,000 | 124 | 165 |
|
*Estimated from sales data Note: AE: adverse event; ADR: adverse drug reaction; MAH: marketing Observation period: December 2009–February 2016 (Cervarix),August |
||||
Other key features of the ADRs reported with HPV vaccines are the diversity of the symptoms and their development in a multi-layered manner over an extended period of time. The ADRs include complex, multi-system symptoms, such as seizures; disturbance of consciousness; systemic pain, including headache, myalgia, arthralgia, back pain and other pain; motor dysfunction, such as paralysis, muscular weakness, exhaustion and involuntary movements; numbness and sensory disturbances; autonomic symptoms, including dizziness, hypotension, tachycardia, nausea, vomiting and diarrhoea; respiratory dysfunction, including dyspnoea and asthma; endocrine disorders, such as menstrual disorder and hypermenorrhoea; hypersensitivity to light and sound; psychological symptoms, such as anxiety, frustration, hallucinations and overeating; higher brain dysfunction and cognitive impairments, including memory impairment, disorientation and loss of concentration; and sleep disorders, including hypersomnia and sudden sleep attacks. In some cases, these symptoms impair learning and result in extreme fatigue and decreased motivation, having a negative impact on everyday life (8, 9, 10, 11). The situation in Japan is similar to that in other countries which have also reported a specific cluster of serious and complex symptoms that develop across multiple body systems over an extended period of time (12, 13).
The reason why HPV vaccines cause these characteristic adverse effects remains to be studied in the future, but one of the most plausible explanations is that these vaccines are designed to maintain an extremely high antibody titre over a long period of time. Since prolonged inflammatory reactions associated with infection are known to cause autoimmune diseases and worsening of autoimmune reactions (14), longtime antigen stimulation with HPV vaccines might also induce complex autoimmune reactions via a mechanism similar to that seen with prolonged infection.
Individuals who experienced ADRs following HPV vaccination established a voluntary liaison organisation to facilitate communication with others who also experienced ADRs in Japan. When these ADRs were reported in the mass media, HPV vaccination became a major social issue. In response to the negative press surrounding HPV vaccination, the MHLW withdrew its active recommendation in June 2013 on the grounds of “an undeniable causal relationship between persistent pain and the vaccination” (1). As a result, the inoculation rate for the vaccine decreased rapidly [from 80% at its peak to less than 1% at present (15)]. In response to this change, proponents of the HPV vaccine initiated a push-back campaign and began actively lobbying the government.
On January 20, 2014, the expert advisory committee established by the MHLW (16) presented the view that the diverse pain and motor dysfunctions experienced by many individuals after HPV vaccination comprised psychosomatic reactions to anxiety or stimulatory pain caused by needle injection, and were not due to any components of the vaccine itself. However, doctors and researchers who examined patients with post-vaccination symptoms arrived at a completely different conclusion, highlighting both the characteristic symptoms and course, which are difficult to explain as psychosomatic reactions (9, 10, 11).
Thus, the safety of the HPV vaccine remains far from certain in Japan, justifying the public’s strong distrust. Recognising the potentially negative influence of these events on public opinion in other countries, pharmaceutical companies initiated a counter-intervention strategy through public and private organisations, such as the World Health Organisation (WHO). The Global Advisory Committee on Vaccine Safety (GACVS), one of the WHO’s advisory committees, claimed it had “not found any safety issue that would alter its recommendations for the use of the vaccine” and criticised the MHLW’s decision to withdraw active recommendation (17).
Despite these obstacles, in July 2016, a victims’ group filed a multi-plaintiff lawsuit in the district courts of Tokyo, Nagoya, Osaka and Fukuoka against the Japanese government and the two pharmaceutical companies that had produced these vaccines. Furthermore, in December of the same year, additional victims joined the multi-plaintiff lawsuit, bringing the total number of plaintiffs to 119 (18).
So far, we have reviewed the adverse reactions to HPV vaccines and the measures taken by the MHLW in Japan that provoked controversy both in Japan and abroad. In the next section, we discuss the safety and efficacy of the HPV vaccines promoted by the WHO and other organisations, and identify a flaw in the basis of their arguments in favour of the vaccines.
Investigation by the MHLW
Regarding Japan, the GACVS statement (17) says that “review of clinical data by the national expert committee led to a conclusion that symptoms were not related to the vaccine”. However, there are major problems with the expert committee’s investigation (16).
The most serious problem is that very few members of the committee actually examined patients with post-vaccination symptoms. The committee’s investigation focused exclusively on pain and motor dysfunction, and ignored many other diverse symptoms that have been observed. Further, cases in which adverse events occurred more than a month after vaccination were excluded from consideration on the ground that most adverse effects of vaccines occur within one month of vaccination. However, subsequent studies have clarified that symptoms commonly appear even after a considerable period of time has elapsed since vaccination (9, 10, 11).
The methods used for determining psychosomatic reactions to be the cause of symptoms are also open to question (16). The expert advisory committee proposed four hypotheses regarding the pathophysiology of post-vaccination symptoms: (i) neurological disorder, (ii) intoxication, (iii) immunological reaction, and (iv) psychosomatic reaction. Those cases which do not conform to the committee’s criteria for (i)–(iii) were regarded as having no causal relationship with the HPV vaccine. However, since the definition of the psychosomatic response is ambiguous and the diagnosis is exclusively made by the subjective judgement of the doctor, many cases are diagnosed as psychosomatic reactions.
Support for the expert advisory committee’s conclusion is far from universal. Doctors and researchers who actually examined patients with post-vaccination symptoms pointed out that it is difficult to explain all symptoms as psychosomatic reactions on the basis of the results of experiments and case reports (8, 9, 10, 11, 20, 21, 22). Prior to investigating HPV vaccine-associated neuro-immunopathy (HANS), a new disease concept proposed by Nishioka (22),Yokota et al excluded from their survey all individuals who exhibited any physical/psychological abnormality before the vaccination (9). Thus, the survey design further strengthened the conclusion that the psychosomatic response could not account for the majority of the AEs of the HPV vaccine, as claimed by the committee.
Further, as 11 of the 15 members of the expert advisory committee have conflicts of interest with vaccine manufacturers, the public is justified in requesting that a more diverse range of scientists reviews the relevant data (23). Thus, the safety of the HPV vaccine remains far from certain in Japan, justifying the public’s strong concerns. Outside Japan, Jefferson et al (24) and Gõtzsche et al (25) also expressed concern about the nature and quality of regulation of the HPV vaccine by the European Medicines Agency.
Criticism of the evidence for safety mentioned in the GACVS statement
Regarding the safety of the HPV vaccine, the GACVS claimed in its statement that it had not found any safety issues warranting an alteration in its recommendations for the use of the vaccine, and criticised Japan for stopping the active promotion of HPV vaccination (17). However, the studies (26, 27, 28, 29, 30, 31) cited by the GACVS as evidence for the vaccine’s safety raise the following fundamental questions.
Among the pathophysiological mechanisms related to adverse reactions after vaccination, the involvement of autoimmunity is one of the most probable. The various mechanisms suggested with regard to autoimmune diseases include: molecular mimicry (32), in which a foreign antigen shares structural similarities with self-antigen; the disruption of essential mechanisms in central and peripheral immune tolerance (33); and human endogenous retroviruses genes producing functional proteins or developing antibodies against the individual’s own proteins (34).
Although the aetiology has not been fully elucidated, most autoimmune diseases are complex polygenic conditions, in which the affected individual inherits multiple genetic polymorphisms that contribute to disease susceptibility, and these genes interact with environmental factors to cause the disease. It is a well-known fact that some human leucocyte antigen alleles occur at a higher frequency in patients with certain autoimmune diseases than in the general population (35).
At present, what is claimed to be the primary evidence for the safety of the HPV vaccine is that there is no statistically significant difference in the incidence of autoimmune diseases among vaccinated females and unvaccinated females or the general population. However, since the proportion of genetically susceptible people in the general population is very small and limited, simple comparisons of the incidence of autoimmune diseases between those who have been vaccinated and a control (unvaccinated) group are likely to show no significant difference. Arguments that do not take this into account cannot assure the safety of the vaccine. The baseline prevalence of many autoimmune diseases is relatively low. Thus, careful large-scale post-marketing surveillance that takes into account the immunological characteristics of individual patients is required to scientifically verify the relationship between vaccination and autoimmune diseases (36).
In drug regulatory agencies and the pharmaceutical industry, all AEs in a patient’s medical record are coded for computer processing and thus, details contained in the raw data are “lost”. As a result, the clinical significance and extent of drug risk are masked (37, 38). This process results in a kind of circular reasoning, in which post-vaccination symptoms are isolated and analysed retrospectively within the framework of the existing disease concepts, instead of being viewed comprehensively.
HPV is equipped with various immune evasion mechanisms, which could cause the immune system to become more tolerant to the infection, creating a microenvironment susceptible to further infection and facilitating the progression of cervical intraepithelial neoplasia (CIN). To counteract these immune evasion mechanisms, the HPV vaccine is designed to maintain an extraordinarily high level of antibodies for more than a decade (39, 40). This moves the HPV vaccine out of the paradigm of “vaccine” as it is conventionally understood. These unique characteristics of the HPV vaccine make it essential to conduct a more thorough evaluation of its safety.
While the GACVS statement claims that “the impact of HPV vaccines on HPV-related clinical outcomes, including pre-cancerous lesions, is well established”, in actuality, the effectiveness of the HPV vaccine is quite limited, as discussed below.
First, the only verified effect of the HPV vaccine is a preventive effect on pre-cancerous lesions (specifically CIN); the preventive effect on cervical cancer itself has not been established. The effects of the vaccines currently approved in Japan (Cervarix and Gardasil) on pre-cancerous lesions have been demonstrated only in the cases of HPV 16 and 18, which, according to the most reliable studies, represent only 50% of cervical cancer cases in Japan (41).
Further, 10% or fewer cases of high-risk HPV infection result in persistent infection that can cause cancer, while the large majority of any pre-cancerous lesions (CIN) that do develop resolve before becoming cancerous (42, 43). Therefore, only 0.15% of individuals infected with high-risk HPV develop (invasive) cancer (44, 45). Even if cancer develops, regular check-ups can help to detect it at an early stage and appropriate treatment (surgery, radiation and drug therapy) saves many lives. On the basis of these facts, the promotion of educational activity that emphasises the importance of screening and early detection, as well as the creation of an environment in which women feel more comfortable undergoing Pap testing, would be far more effective at preventing cervical cancer than would pressuring teenage girls to receive the existing HPV vaccination, with all its problems.
The proponents of the HPV vaccines claim that they are 98% – 100% effective in preventing cervical cancer. In reality, however, the absolute risk reduction (ARR) provided by HPV vaccines is, at most, 0.1% – 0.7%, on the basis of calculations using the existing data (46). Further, this indicates only the reduction in the risk of developing pre-cancerous lesions, while the risk of developing cervical cancer remains unknown.
The promotion of screening for cervical cancer is another important measure against cervical cancer. For a long time now, attention has been drawn to the low screening rate for cervical cancer in Japan compared to the western countries. In particular, young women with no experience of pregnancy are reluctant to undergo gynaecological examinations in Japan. Access to examinations by female doctors and an acceptance of self-sampling would undoubtedly increase the screening rates. In fact, the promotion of screening for cervical cancer significantly reduced the age-adjusted incidence of invasive cervical cancer in the UK (47).
In the previous sections, we discussed various issues regarding the safety and effectiveness of the HPV vaccine. It is now appropriate to ask how such questionable vaccines have come into widespread use. The answer, at least with respect to Japan, can be found in a structural flaw, combined specifically with the following factors: (i) aggressive promotion by the pharmaceutical industry, (ii) trade negotiations by economic superpowers, and (iii) contemporary medicine, which is characterised by overconfidence in technology and a lack of humility with respect to listening to patients’ complaints.
| Table 2: Vaccination and legal categorisation | ||
| Category | Responsibility of individual | Vaccination |
| Routine vaccination A | Duty to make effort to receive vaccination | Hib, pneumococcal, BCG, diphtheria, pertussis, tetanus, polio, measles, rubella, varicella, HPV, HB, Japanese Encephalitis |
| Routine vaccination B | No particular social duty | Influenza (for elderly), pneumococcal |
| Optional vaccination | Discretion of individual | Pneumococcal (for adults), rotavirus,etc. |
Following the enactment of the Immunisation Act in Japan in 1948, numerous lawsuits were filed in response to vaccine related injuries. This resulted in the establishment of a compensation system for victims and the amendment of the relevant laws and regulations. At present, vaccines are divided into three categories, as shown in Table 2 (48).
According to the definitions in the Act, a vaccine for individual protection, such as the HPV vaccine, should be classified as an “optional” vaccination, which is solely the individual’s choice. However, due to lobbying activities, the HPV vaccine was approved as a vaccine to be administered at public expense, and was included in the category “Routine vaccination A”. Since it was recommended by the government, individuals felt obligated to receive the HPV vaccine.
The Japanese Expert Board for the Eradication of Cervical Cancer (49), one of the most powerful lobbying organisations in Japan, was founded in November 2008, around the time the HPV vaccine was being reviewed for approval. The executive members of various medical academic societies joined this group and exerted considerable influence on the legislative process, as well as on public administration and the shaping of public opinion.
According to information obtained by Medwatcher Japan (50) under the Transparency Guideline for the Relation between Corporate Activities and Medical Institutions (51) of the Japan Pharmaceutical Manufacturers Association, the funds received by the Expert Board from vaccine manufacturers amounted to ¥73,500,000 (¥35,000,000 in 2012 and ¥38,500,000 in 2013). In addition, the secretary of the Expert Board was found to have been working at GlaxoSmithKline Co. as the Director of Marketing for vaccines for up to eight months prior to the launch of Cervarix. These facts strongly suggest that the activity of the Expert Board was not altruistic, but was actually disguised promotion (52).
The promotion of the HPV vaccine during Japan–US trade negotiationshas also created pressure on Japan to adopt the vaccine. For many years, the promotion of vaccination has been one of the most pressing requirements in trade negotiations with the US, Japan’s most important trading partner (53, 54). The Center for Strategic and International Studies, a civilian think tank that is part of the US military–industrial complex, criticised the indecisiveness of Japan’s government in reports issued in May 2014 and April 2015, reflecting the irritation of US industries (55, 56).
Basic defects inherent in the medical community underlie the issue of the HPV vaccine. In 2004, Sheldon Krimsky pointed out the increasing influence of commercialism in academic science and biomedical research in his book, Science in the private interest (57). He wrote, “…the mix of science and commerce continues to erode the ethical standards of research and diminish public confidence in its results. “In the 13 years since the publication of the book, his warning has become a reality everywhere in the world, not only in the USA. Originally, public health and pharmaco-epidemiology were the scientific fields that aimed to protect the health of individual patients and the public. However, the current reality is very far from the ideal.
Science is now misused to protect the interests of the pharmaceutical industry, and has been used to deny the causal relationship between the drug and its adverse reactions. Many researchers and experts are attempting to exclude inconvenient truths from consideration. “The taxonomy of diseases represents the nearest science has got to nature, but it remains a theoretical construct. It is the theory that should be discounted when the patient’s symptoms refuse to fit, not the patient’s account of the reality of their experience.” (58, 59) This means that doctors must be more humble and scientifically honest. Today’s diagnostics and therapeutics were created by listening to patients’ voices and conducting careful examinations. It is irresponsible to dismiss a patient’s complaint as a psychogenic reaction or a general phenomenon among young women without conducting a thorough examination.
As described in section III, the introduction of HPV vaccination in Japan was promoted with an emphasis on commercial interests rather than as a public health need. This situation is not unique to Japan and has also been observed in other countries. In Australia, for example, despite the considerable doubts of the Pharmaceutical Benefits Advisory Committee about the Gardasil vaccine, the committee’s decision to reject the addition of Gardasil to the national vaccination schedule was hurriedly overturned, following political interference and lobbying by other vested interests (60). In the USA, Merck & Co, Inc promoted legislation to mandate HPV vaccination for school attendance by serving as an information resource, lobbying legislators, drafting legislation, mobilising female legislators and physicians’ organisations, conducting consumer marketing campaigns, and filling gaps in access to the vaccine. Legislators relied heavily on Merck for scientific information (61).The responsibility to prove the efficacy and safety of a vaccine lies with the pharmaceutical companies, and the government is expected to monitor and guide these efforts. The current situation in which commercial interests drive government policy must be corrected from a medical ethics perspective.
At present, Japan is one of the few countries in which the active recommendation of HPV vaccination has been temporarily stopped; the regulatory authorities in other countries have not changed their policies. Although various groups of victims of vaccination have collaborated on wideranging activities in these countries, the regulatory authorities have not yet admitted the causal relationship between the vaccines and the victims’ health injuries.
The Japanese government’s decision to stop actively recommending HPV vaccination has, to an extent, encouraged regulators and patients in other countries to question the value of HPV vaccination. Japan’s efforts to stop active recommendation might have been successful because of its historical background of cases of environmental pollution and drug-induced suffering (Minamata disease, thalidomide, SMON, dura mater graft-associated Creutzfeldt–Jakob disease, HIV transmitted by contaminated blood products, etc), which occurred during the post-war period of rapid economic growth. In the multi-plaintiff suits that followed the instances of environmental pollution and drug-induced suffering, the plaintiff groups sought not only compensation for damages, but also institutional reform and revisions to the law to prevent the repetition of the same mistakes (62).
This historical background has created a situation in which the mass media and regulators cannot easily ignore the victims’ complaints about the side-effects of new vaccines. It is here that we may find a clue on how to solve this problem. It is necessary to enhance transparency at every step of the approval process for pharmaceutical products, from new-drug development to post-marketing surveillance. At the same time, it is crucial to strengthen the management of conflicts of interest, and develop a system by which citizens can participate directly and have a voice in the planning of public health policy (63, 64, 65).
Conflict of interest
All the authors are members of Medwatcher Japan. Masumi Minaguchi and Masato Sekiguchi are Lawyers for the plaintiffs in the HPV vaccination lawsuits.
References
- Notification from MHLW on routine vaccination programme of HPV vaccine 2013.6.14 [Japanese] [cited 2017 Mar 25]. Available from: http://www.mhlw.go.jp/stf/shingi2/0000091963.html
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite guideline Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A [cited 2017 Mar 25]. Available from: https://www.imim.es/media/upload/arxius/MEDIA436.pdf
- Documents 16&17 distributed at the meeting of Council of Health Sciences, subcommittee of vaccination, ADR Working group meeting, May 23, 2016 [ Japanese][cited 2017 Mar 25]. Available from: http://www.mhlw.go.jp/stf/shingi2/0000125164.html
- Documents distributed at the meeting of Council of Health Sciences, subcommittee of vaccination, ADR Working group meeting, April 12, 2016 [Japanese] [cited 2017 Mar 25]. Available from: http://www.mhlw.go.jp/stf/shingi2/0000121045.html
- Documents distributed at the meeting of Council of Health Sciences, subcommittee of vaccination, ADR Working group meeting, May 23, 2016 Japanese][cited 2017 Mar 25]. Available from: http://www.mhlw.go.jp/stf/shingi2/0000125164.html
- Lawrence G, Gold MS, Hill R, Deeks S, Glasswell A, McIntyre PB. Annual report: Surveillance of adverse events following immunisation in Australia, 2007. Commun Dis Intell Q Rep. 2008 Dec;32(4):371-87.
- National Vaccine Information Center. An Analysis by the National Vaccine Information Center of Gardasil & Menactra Adverse Event Reports to the Vaccine Adverse Events Reporting System (VAERS), February 2009[cited 2017 Mar 25]. Available from: http://www.cbsnews.com/htdocs/NVICGardasilvsMenactraVAERSReportFeb2009.pdf
- Kinoshita T, Abe RT, Hineno A, Tsunekawa K, Nakane S, Ikeda S. Peripheral sympathetic nerve dysfunction in adolescent Japanese girls following immunization with the human papillomavirus vaccine. Intern Med. 2014;53(19):2185-200.
- Yokota S, Kuroiwa Y, Nakamura I, Nakajima T, Nishioka K. General overview and discussion on HPV vaccine associated neuropathic syndrome. Japan Medical Journal (Nihon Iji Shimpou) 2015;4758:46-53 [Japanese].
- Hirai T, Kuroiwa Y, Hayashi T, Uchiyama M, Nakamura I, Yokota S, Nakajima T, Nishioka K, Iguchi Y. Adverse effects of human papilloma virus vaccination on central nervous system. The Autonomic Nervous System. 2016;53:49-64.
- Ikeda S. Neurological complications in HPV vaccination. Brain and Nerve 2015;67(7):835-43 [Japanese].
- Tomljenovic L, Shaw CA. Human papillomavirus (HPV) vaccine policy and evidence-based medicine: are they at odds? Ann Med. 2013 Mar;45(2):182-93. doi: 10.3109/07853890.2011.645353.
- Brinth L, Theibel AC, Pors K, Mehlsen J.Suspected side effects to the quadrivalent human papilloma vaccine. Dan Med J. 2015;62(4):A5064.
- Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, Bassetto F, Doria A.Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2010 Mar;87(3):385-95. doi: 10.1189/jlb.0709517. Epub 2009 Dec 16.
- Immunization coverage rates in Japan [cited 2017 Mar 25]. Available from: http://www.mhlw.go.jp/topics/bcg/other/5.html
- Conference Minutes of Council of Health Sciences, subcommittee of vaccination, ADR Working group meeting, January 20, 2014 [Japanese] [cited 2017 Mar 25]. Available from: http://www.mhlw.go.jp/stf/shingi2/0000091998.html
- Global advisory committee on vaccine safety: statement on safety of HPV-vaccines, December 17, 2015 [cited 2017 Mar 25]. Available from: http://www.who.int/vaccine_safety/committee/GACVS_HPV_statement_17Dec2015.pdf?ua=1
- Plaintiffs Lawyers of HPV Vaccines Lawsuits [cited 2017 Mar 25]. Available from: https://www.hpv-yakugai.net/
- Medwatcher Japan. Submission of “Refutation of GACVS (Global Advisory Committee on Vaccine Safety) statement on Safety of HPV vaccine on December17, 2015”, November 2016 [cited 2017 Mar 25]. Available from: http://www.yakugai.gr.jp/en/topics/topic.php?id=930
- Takahata K, Takashima H. A proposal for a new neurological examination for discrimination of autoimmune encephalopathy and somatoform disorders. Neurological Therapeutics. 2016;33(1):9-18 [Japanese].
- Aratani S, Fujita H, Kuroiwa Y, Usui C, Yokota S, Nakamura I, Nishioka K, Nakajima T. Murine hypothalamic destruction with vascular cell apoptosis subsequent to combined administration of human papilloma virus vaccine and pertussis toxin. Sci Rep. 2016 Nov 11;6:36943. doi: 10.1038/srep36943.
- Nishioka K, Yokota S, Matsumoto Y. Clinical features and preliminary diagnostic criteria of human papillomavirus vaccination associated with neuroimmunopathic syndrome (HANS). Int J Rheum Dis 2014;17(suppl 2): 6-29.
- Medwatcher Japan: Submission of a “Request to reconsider the rules on conflict of interest (COI) for Ministry of Health, Labour and Welfare councils – In light of the COI issues with council members regarding HPV vaccines”, April 2014 [cited 2017 Mar 25]. Available from: http://www.yakugai.gr.jp/en/topics/topic.php?id=863
- Jefferson T, Jõrgensen L. Human papillomavirus vaccines, complex regional pain syndrome, postural orthostatic tachycardia syndrome, and autonomic dysfunction – a review of the regulatory evidence from the European Medicines Agency. Indian J Med Ethics 2017;2(1):30-37.
- Gõtzsche PC, Jõrgensen KJ, MD, Jefferson T, Auken M, Brinth L. Complaint to the European ombudsman over maladministration at the European Medicines Agency (EMA) in relation to the safety of the HPV vaccines, October 10, 2016, [cited 2017 Mar 25]. Available from: http://nordic.cochrane.org/sites/nordic.cochrane.org/files/public/uploads/ResearchHighlights/Complaint-to-ombudsman-over-EMA.pdf
- Agence nationale de sécurité du medicament et des produits de santé. Vaccins anti-HPV et risque de maladies autoimmunes: etude pharmacoépidémiologique [French]. [cited 2017 Mar 25]. Available from: http://ansm.sante.fr/content/download/80841/1023043/version/1/file/Ansm_gardasil-Hpv2_Rapport_September-2015.pdf
- Rasmussen TA, Jõrgensen MR, Bjerrum S, Jensen-Fangel S, Stõvring H, õstergaard L, Sõgaard OS.Use of population based background rates of disease to assess vaccine safety in childhood and mass immunisation in Denmark: nationwide population based cohort study. BMJ. 2012 Sep 17;345:e5823. doi: 10.1136/bmj.e5823.
- Arnheim-Dahlström L, Pasternak B, Svanström H, Sparén P, Hviid A.Autoimmune, neurological, and venous thromboembolic adverse events after immunization of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013 Oct 9;347:f5906. doi: 10.1136/bmj.f5906.
- Callréus T, Svanström H, Nielsen NM, Poulsen S, Valentiner-Branth P, Hviid A.Human papillomavirus immunization of adolescent girls and anticipated reporting of immune-mediated adverse events. Vaccine. 2009 May 14;27(22):2954-8. doi: 10.1016/j.vaccine.2009.02.106. Epub 2009 Mar 13.
- Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, Dubin G.Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin. 2009;5(5):332-40.
- Chao C, Klein NP, Velicer CM, Sy LS, Slezak JM, Takhar H, Ackerson B, Cheetham TC, Hansen J, Deosaransingh K, Emery M, Liaw KL, Jacobsen SJ. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271(2):193-203.doi: 10.1111/j.1365-2796.2011.02467.x. Epub 2011 Nov 15.
- Cusick MF, Libbey JE, Fujinam RS. Molecular Mimicry as a Mechanism of Autoimmune Disease. Clin Rev Allergy Immunol. 2012;42(1):102-11.
- Marson A, Housley WJ,Hafler DA. Genetic basis of autoimmunity. J ClinInvest. 2015;125(6):2234-41.
- Volkman HE, Stetson DB. The enemy within: endogenous retroelements and autoimmune disease. Nat Immunol. 2014;15(5):415-22.
- Abbas AK, Lichtman AH, Pillai S. Immunologic tolerance and autoimmunity. In: Abbas AK, Lichtman AH, Pillai S (eds). Cellular and Molecular Immunology, 8th ed. Philadelphia: Elsevier Saunders; 2015, pp.315-337.
- Castiblanco J, Anaya JM. Genetics and vaccines in the era of personalized medicine. Curr Genomics. 2015 Feb;16(1):47-59. doi: 10.2174/1389202916666141223220551.
- Healy D. Doctoring the data. In: Pharmageddon. Berkeley and Los Angeles: Univ. of California Press; 2012,pp.96-128.
- Herxheimer A. Pharmacovigilance still neglects patients. The Informed Prescriber. 2014;29(5):75-9 [Japanese].
- Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, Lalezari J, David MP, Lin L, Struyf F, Dubin G; HPV-010 Study Group.Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a Phase III randomized trial. Hum Vaccin Immunother. 2014;10(12):3435-45. doi: 10.4161/hv.36121.
- Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, Zahaf T, Catteau G, Geeraerts B, Descamps D.Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. HumVaccin Immunother. 2014;10(8):2147-62. doi: 10.4161/hv.29532.
- Asato T, Maehama T, Nagai Y, Kanazawa K, Uezato H, Kariya K. A large case–control study of cervical cancer risk associated with human papillomavirus infection in Japan, by nucleotide sequencing-based genotyping. J Infect Dis. 2004 May 15;189(10):1829-32. Epub 2004 Apr 26.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998 Feb 12;338(7):423-8.
- Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, Yates M, Rollason TP, Young LS.Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357(9271):1831-6.
- Kawana K,Yasugi T. Human papillomavirus and neoplastic disorder. Antibiotics & Chemotherapy. 2006;22(10):1521-8 [in Japanese].
- Department of vaccines and other biologicals. The current status of development of prophylactic vaccines against human papillomavirus infection. Report of a technical meeting, Geneva, February 16-18, 1999.
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374 (9686):301-14. doi: 10.1016/S0140-6736(09)61248-4. Epub 2009 Jul 6.
- Quinn M, Babb P, Jones J, Allen E.Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics. BMJ. 1999;318(7188):904-8.
- Immunization Act [cited 2017 Mar 25]. Available from: http://www.japaneselawtranslation.go.jp/law/detail/?id=2778&vm=04&re=01
- The Japanese Expert Board for the Eradication of Cervical Cancer [cited 2017 Mar 25]. Available from: http://www.cczeropro.jp/
- Medwatcher Japan. Complaint against HPV vaccine manufacturers’ alleged violations of the JPMA Promotion Code for Prescription Drugs [Japanese] [cited 2017 Mar 25]. Available from: http://www.yakugai.gr.jp/en/topics/topic.php?id=890
- Transparency guideline for the relation between corporate activities and medical institutions, The Japan Pharmaceutical Manufacturers Association (JPMA) [cited 2017 Mar 25]. Available from: http://www.jpma.or.jp/english/policies_guidelines/transparency_guideline.html
- Complaint against HPV vaccine manufacturers’ alleged violations of the JPMA Promotion Code for Prescription Drugs[cited 2017 Mar 25]. Available from: http://www.yakugai.gr.jp/en/topics/topic.php?id=890
- Annual Reform Recommendations from the Government of the United States to the Government of Japan under the U.S.-Japan Regulatory Reform and Competition Policy Initiative October 15, 2008[cited 2017 Mar 25]. Available from: https://www.google.com/url?q=https://ustr.gov/sites/default/files/uploads/agreements/morocco/pdfs/EHI%2520USG%2520Agenda%2520Items%25202-11-11%2520FINAL.pdf&sa=U&ved=0ahUKEwio496zzdDSAhWKH5QKHa4BDFoQFggEMAA&client=internal-uds-cse&usg=AFQjCNEf8amvE_-1ixWyJPGZcjmHAyLGaQ
- United States-JAPAN. Economic Harmonization Initiative, February 2011 [cited 2017 Mar 25]. Available from: https://www.google.com/url?q=https://ustr.gov/sites/default/files/2008-2009-Regul
- Wilson R, Paterson P, Larson HJ. The HPV vaccination in Japan – issues and options. CSIS, May 2014[cited 2017 Mar 25]. Available from: https://csis-prod.s3.amazonaws.com/s3fs-public/legacy_files/files/publication/140514_Wilson_HPVVaccination_Web.pdf
- Wilson R, Paterson P, Chiu J, Schulz W, Larson H. HPV vaccination in Japan — the continuing debate and global impacts. CSIS, April 2015 [cited 2017 Mar 25]. Available from: https://csis-prod.s3.amazonaws.com/s3fs-public/legacy_files/files/publication/150422_Wilson_HPVVaccination2_Web.pdf
- Sheldon Krimsky. Science in the private interest: has the lure of profits corrupted biomedical research? Oxford: Rowman & Littlefield Publishers, Inc; 2003, p4.
- Heath I.Following the story:continuity of care in general practice. In: Greenhalgh T, Hurwitz B (eds). Narrative based medicine. London: BMJ Books; 1998, pp.86.
- Rudebeck CE. Humanism in medicine. Benevolence or realism? Scand J Prim Health Care. 1992 Sep;10(3):161-2.
- Hart E. The history of questionable fast-tracked global HPV vaccination [cited 2017 Mar 25]. Available from: https://elizabethhart.files.wordpress.com/2013/02/the-history-of-questionable-fast-trackedglobal-hpv-vaccination.pdf
- Mello MM,Abiola S, Colgrove J. Pharmaceutical companies’ role in state vaccination policymaking: the case of humanpapillomavirus vaccination. Am J Public Health. 2012 May;102(5):893-8. doi: 10.2105/AJPH.2011.300576. Epub 2012 Mar 15.
- Suzuki T, Minaguchi M, Sekiguchi M. Law and safety of drug. Eidell Institute; 2015,p.348 [Japanese].
- Chalmers I. What do I want from health research and researchers when I am a patient? BMJ. 1995;310(6990):1315-18.
- Doshi P, Dickersin K, Healy D, Vedula SS, Jefferson T. Restoring invisible and abandoned trials: a call for people to publish the findings. BMJ. 2013 Jun 13;346:f2865. doi: 10.1136/bmj.f2865.
- Beppu H. Reasons why patients should take part in the planning of clinical trials. The Informed Prescriber 2010;25(4):45-9 [Japanese].
