ARTICLES
Ethical challenges in biobanking: moving the agenda forward in India
Manjulika Vaz, Mario Vaz, Srinivasan K
DOI: https://doi.org/10.20529/IJME.2014.022
Abstract
There is no agreement on the typology and definition of biobanks. The present regulations across countries, including India, focus on genomic and genetic databases and DNA and cell line biobanking. It is unclear how the range of the holdings of biological samples in diagnostic and research laboratories fall under these regulatory frameworks. Biobank-related research has become very attractive because of advances in sample storage and data processing, a better understanding of the human genome, and high throughput laboratory assays. There is extensive literature and much debate on the subject, especially on the ethical and regulatory dilemmas, in the developed countries, but this is hardly the case in developing countries. This paper is based on a review of the published documents and data, and aims at evaluating the ethical frameworks for biobanking in the Indian context. The issues of “broad consent”, commercialisation of samples, and extended sample use are discussed. The governance of biobanks emerges as an integral part of the ethical responsibilities of institutions. It also makes the implementation of national guidelines possible, and helps to enhance the trust and confidence of local contributors in biobank research.
Introduction
The multiple uses of old samples (1), commercialisation of genetic material, private gain versus public good and possible sharing of benefits (2, 3, 4) have all emerged as various aspects of the growing debate on biobanking and biomedical research. The assumption that biobanks are neutral storage places or repositories of knowledge appears problematic and justifies the scope of the ethical debate (5).
There has been relatively little discourse on the possible ethical issues related to biobanking in India. This paper reviews studies in the broad area of ethics and biobanking from around the world and raises issues that may be of relevance to India. Country-specific guidelines and regulations help to address ethical issues in a local cultural context, and these are constantly evolving across the world as experiences change and evidence is gathered. Apart from regulations, mechanisms for oversight are needed to hold investigators, institutions and investors accountable and to monitor compliance with the regulations.
Definition of a biobank
While human tissue has been collected for at least a hundred years (6), the word “biobanking” first appeared in PubMedin 1996 (7). However, even today there is no universally agreed upon definition of a biobank. Thus, while some consider a biobank “a large-scale collection of human biological material” (8), it may also refer to smaller collections of tissue (pathology paraffin blocks, biological fluids such as blood and urine, surgically removed “waste” tissue, and so on) stored in diagnostic departments, not with the primary aim of conducting research, but rather, of informing treatment (9, 10). Samples and data stored by the pharmaceutical industry that have a potential for commercial gain would also constitute a biobank (11, 12). In addition, the medical, genealogical and lifestyle-related data linked with the biological samples are considered a part of the biobank and are as important as the samples (9, 13, 14). Residual samples from earlier clinical trials or research studies stored as formal or informal collections in an individual researcher’s freezer would also constitute a biobank (13). The other terms in use for a human tissue biobank are “biorepository”, “biolibrary” and “biospecimen resource”.
The 2006 Indian Council of Medical Research (ICMR) guidelines defined a biobank/biorepository as “a collection of resources that can be accessed to retrieve human biological material and data” (15:p71). While broad enough to include all collections of tissue/blood and data, this definition is placed under the heading of “DNA, cell line banking” in the chapter on Human Genetics and Genomics Research, whichsuggests a more focused and limited scope. The ICMR’s concern with genetic information is understandable, given that Article 20 of the UNESCO Draft Declaration on Human Genetic Data urges that “States may consider establishing a framework for the monitoring and management of human genetic data, human proteomic data and biological samples based on the principles of independence, multidisciplinarity, pluralism and transparency, as well as the principles set out in Declaration…” (8).
It would perhaps be appropriate at this stage for the ICMR to widen the scope of its guidelines to include other “non-genetic” uses of biobanks, given that the scope of research on stored biological samples is growing and also, so that investigators using stored samples for other research may have a clear idea of the ethical guidelines that they need to adhere to. In the absence of this, individuals, researchers and laboratories will either opt in or out of the biobanking regulations on the basis of their interpretation of the existing guidelines.
Typology of biobanks
Drawing upon the varied descriptions of biobanks worldwide, we have made an attempt to describe a broad typology of biobanks, as follows.
Table 1 Typology of biobanks
| Based on use/coverage
General/population (UK Biobank, Estonian Biobank) Disease-specific (brain biobank, cancer biobank, atherosclerosis biobank) |
Reference
13, 9 16 |
| Based on source of funding/governance
Public (HUNT Biobank, Norway, genome analysis of EU twins, Finland) Private (Iceland’s deCODE biobank, Estonia’s EGeen Inc) Public-private partnerships (UK Biobank, GRAD USA) |
17, 9 18,9 19, 9 |
| Based on type of sample and its source
Clinical setting: tissue samples in pathology laboratories, collections of screening data cards, etc Research projects: samples from epidemiological studies Judiciary domain: DNA fingerprints, other human biological material/data Pharmaceutical industry: tissue extracts, cell lines, clinical data |
9 9 9 11 |
| Based on time/period of collection
De novo (prospective) collections (Iceland biobank, Estonian Gene Bank, UK Biobank) Historical (retrospective) collections: pathology archives in hospitals, newborn screening cards |
10 10 |
| Based on size/degree of access
Investigator-driven (single focused study) University (multiple investigators) Regional National (eg Iceland decode biobank, Estonian Biobank, UK Biobank) International (shared biobank across countries – EuroBioBank, GenomEUtwin |
9 20, 9 |
This typology reflects the immense possibilities for research at a micro or macro level, with the potential for collaboration. It also indicates the possible complexities of organising, managing and governing these collections of human biological material, as well as the diversities that would need to be dealt with, and emphasises the eminent need for standardised international legislation on the one hand, and localised, specialised frameworks on the other. There have also been calls for a global registration of biobanks (16).
Why is biobanking so attractive?
As newer technologies have emerged, allowing small amounts of biological material to be analysed in greater detail than ever before, and as advances in biotechnology, bio-informatics and genomics have improved our ability to handle large datasets (21), the quest for the discovery of therapeutic biomarkers and for molecular mechanisms to explain the causes of diseases, as well as for more personalised therapeutic options continues (5, 22, 16). In the developed countries, where biobanking developed earlier, the commercial potential of new drugs and treatment regimens has led industry to get increasingly involved in the establishment and development of biobanks, either in conjunction with the government or on their own (9, 19, 23). Many governments and economists are also looking to the findings of biobank-related research to predict disease patterns to guide health policy and health expenditure (22, 24). The issue of the usefulness of such research has not escaped the developing countries, particularly as large population studies that are currently under way allow the linking of clinical and other phenotypic data with biological markers (25).
On the other hand, there is also discussion on how biobanking research may not be able to deliver on all that it claims and it is asserted that a number of studies are “fishing expeditions” (5). Clearly, the utility of biobanking is not universally accepted.
Ethical issues in relation to biobanking
Biobank-related research involves several unique issues with ethical implications, even though it has been argued that population biobanks are associated with little or no risk as they “do nothing” to harm the body of an individual. While biobanks may effectively “house” the samples and accompanying data of the donors, the utilisation of the samples may involve multiple investigators and institutions, both academic and commercial, and there is thus a need for all these players to follow the same ethical frameworks (5).
In order to discuss the ethical issues related to biobanking, we have worked within a broad framework that is summarised in Figure 1.
In this schema, ethical issues are assessed under three broad categories:
- The health needs of the people and the participants in the research process
- The research process itself
- The outcomes of the research process
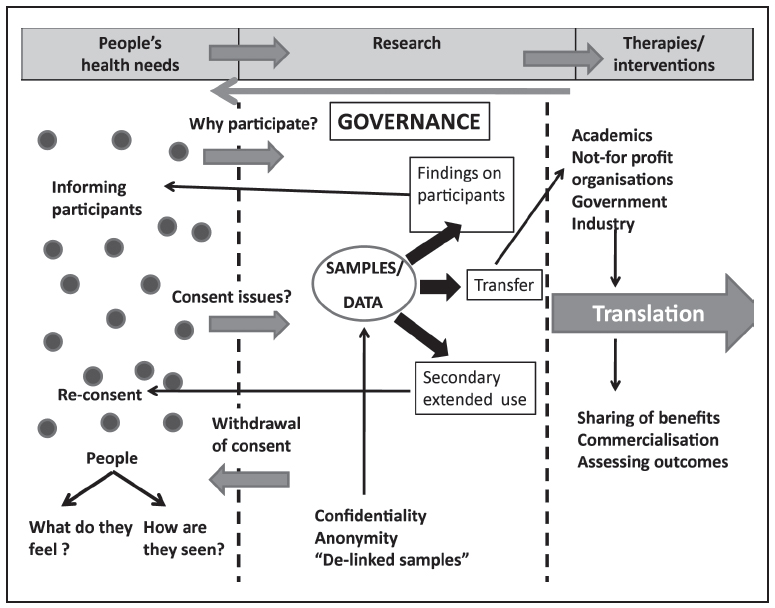
This schema is based on the simple reasoning that research should be driven by the health needs of the people and draws on participants who are part of that population. Biomedical research is not meant to be just scientifically relevant, but should have societal relevance as well (26). Apart from addressing societal concerns, the actual research process needs to be driven by core values that reflect a high level of understanding of ethical ramifications. Public perceptions of the ethical issues related to biomedical research are important since ethical research should address public concerns. These have, in fact, become institutionalised during the formation of many biobanks (2, 6, 27). Finally, translation of research should ideally arrive at outcomes which address the health needs of the people and which are driven by the principles of justice and common good.
-
Ethical issues related to the health needs of people and the participants in the research process
- Trust in the institution collecting their sample, in addition to trust in their country’s legal and regulatory systems, is the primary reason for participating (13, 17, 19, 28, 29).
- An ethnographic study of lay people in British Columbia, Canada revealed that participation was due to altruistic motives, such as “the availability of medical breakthroughs to poorer countries” and the wish “that biobanks move towards universal biolibrary concepts” (6).
- In Scotland, people whose genetic data was being stored in the national genetic biobank participated for “the future of society” and the “common good” (2).
- Another motivation is the expectation of personal gain in terms of health (2, 6). Participants often believe that a treatment option or improved drug will be provided (“therapeutic misconception”) (12), and sometimes even expect to get a free health check-up (19).
- The willingness to participate is greater especially in the case of disease-specific sample contribution and disease-specific research (2, 19).
- Lay people sometimes express fears about “researchers overstepping limits or regulations”, “genetic modification”, eugenics, and researchers violating their privacy by “playing God” (6, 18, 29).
- “Gene angst”, ranging from concerns about “genetic reductionism”, ie being reduced to a molecular entity, to fears about the abuse of genetic information, unethical and “fascist applications” of genetic knowledge and subsequent stigmatisation by insurance companies, the police and the courts, and a decline of “social solidarity” may be important (10, 13, 30).
- There are fears with respect to commercial interest, investment by industry and venture capital in DNA sequencing and the possibility of genetic information becoming the “currency of the future” (9, 10, 18, 30).
-
Ethical issues related to the research process
-
Issues related to translation of research
What do people think about biobank-related research and why do they participate?
People participate in research for a variety of reasons and it is important to understand these, together with their ethical implications. Both quantitative surveys and qualitative studies on participants in biobank research and prospective participants in proposed biobank research in various developed countries show that the public has mixed perceptions regarding biobanking. The qualitative studies, however, appear to provide deeper insights into the reasons for participation/non-participation of the participants (19). Some of these are listed below.
In developing countries, public perceptions of biobanking are largely unexplored. Socioeconomic factors, such as the high level of poverty, low literacy rate, caste and class hierarchies, gender inequality, issues related to power, and traditional practices, play a key role in voluntary participation in medical research. In India, some studies on the reasons for participation in clinical trials have reported altruism as the primary motive (31, 32), followed by the expectation of receiving health benefits (31), the implicit faith that doctors would do no harm (31, 32), and respect for doctors and indebtedness towards them (31). The issue of faith in the physician and the conflict between the physician’s roles as a physician and a researcher (12) may be particularly important in countries such as India, where the treating physician wields considerable power in the doctor-patient relationship and access to health services is limited. Health benefits such as a free health check-up and “therapeutic misconception” (12) would also be important ethical issues in India, where access to health services is poor and the expectation of immediate health benefits is genuine. Biobank-related research may hold real benefits for the participants since baseline diagnostic tests, which are considered “routine” abroad, may provide information to the patient that could result in actionable responses, eg a simple blood glucose test may uncover a significant number of diabetics in a population which does not routinely get screened. Therefore, the ethical questions that need to be asked in the context of biobanking are what good purpose is being served for the population from which the samples are being collected, whether the population’s immediate health needs are being met in any way, and what implications this may have for participation.
How are “people” seen in biobanking research?
The views of “the establishment” or the medical fraternity, ie researchers, academics, scientists, investors, etc, are also important in determining the ethical underpinnings of biobanking. The descriptions used for those who participate in biobank-related research convey important messages about how people are viewed in biobanks and give us an idea of the researchers’ conceptual constructs regarding participants. In his vast review of the literature on biobanking ethics, Klaus Hoeyer (19) takes stock of the relevant debate over the terms used and the relationship between researchers and subjects. The most common term used is “donor, which denotes one who gives his/her tissue as a gift or donation, with no expectation of anything in return. Others use the term “participant,” which suggests a more active involvement in the process of research (this raises the issue of whether active involvement is possible in biobank research and if the use of the term puts too much pressure on people to remain involved). “Samples” and “sources” are more neutral, non-committal terms, which conveniently view the person, whether a patient or a research volunteer, more as a “source” than an individual (21). The term “contributors,” which is used by a few people, acknowledges that the sample given by the person is a contribution to research and that the researcher recognises this (19, 21, 33).
There is an ongoing debate among researchers about their perception of participants. This is perhaps best exemplified by the discussion on Hendrietta Lacks (34), who lost her life in 1951 to cervical cancer, but whose cells (the HeLa cell lines) continued to be used in laboratories and exploited by industry without the knowledge of her immediate family for many years (34). A similar situation was faced by John More, whose cancerous spleen cells were extracted and worked upon as the “Mo cell lines” in the 1980s. More said that it was “very dehumanising to be thought of as Mo… I was not the person … I was the cell line, like a piece of meat” (34).
Regulations also reflect ethical positions. The ICMR guidelines of 2006 state that “research on banked human tissue samples is conducted in a laboratory and hence does not directly involve the individual” (15). While this is technically correct, it does convey how the guidelines perceive of the person contributing his/her sample for biobanking research.
Consent of contributors in biobanking research
It is often argued that because there is little or no risk of physical harm to the contributors of samples/data, explicit individual consent is not required (17). However, all good research is expected to uphold the universal principle of “voluntariness” in the matter of participation in medical research or scientific experimentation. Voluntariness is the freedom to participate and the freedom to withdraw, and this is enforced through the practice of obtaining “informed consent”. This is in line with the principle of respect for the dignity, self-determination and recognition of the contribution of the person involved (33). In biobanking, the term “informed consent” may actually be a misnomer as true informed consent is that given for a well-defined scientific study with specific endpoints, whereas the aims of biobank-related research may not be decided clearly at the time that the sample is provided (9, 17, 19). There is usually a time lag between the collection of the sample and its use in research. Another complex issue with respect to consent in biobanking research is that there are multiple stages and possibilities for which consent needs to be obtained – storage, interventions/tests on the sample, the use of data linked to the sample, whether or not one should be informed about the findings of the research, transfer of the sample/data to other investigators/institutions, possible commercialisation and the possibility of deriving financial benefit from the samples/data and all that this involves.
Varied forms of consent have been devised to deal with all these problems. Some allow for delayed use and some for secondary use of the stored sample in a biobank/biorepository. “Blanket consent” is the most general consent, with the participant not knowing the specific purpose of the research. Some argue that this can hardly be considered consent (9, 19, 35). In the case of “presumed consent,” the donor receives information on the details of the study, and if no response is received, it is considered “presumed” or “passive” consent (17). There is considerable debate on the extent to which passive consent meets ethical standards (9, 17, 36).It could also lead to anger and mistrust (13). The use of “broad consent” or “enlarged consent”, which is in vogue in the de novo biobanks (UK and Iceland), has been proposed when the specific use of biological samples is not known but consent is sought for their future use, while providing a broad framework for their potential use (36). “Group consent” or “community consent” is another option, especially for genetic studies. Here, the possible outcomes and the scope of the work are discussed with the family/community before individual consent is obtained (9, 19).There is considerable uncertainty about whether consent is needed at all for research on residual samples, especially microbiological samples. Bacterial growths on culture can be used for a variety of research purposes and do not constitute human samples, yet are derived from human beings. In such situations, research carried out on the “sample” can potentially be linked to the individual’s data. Some would argue that such linkage is, indeed, critical for research. A recent paper suggests that in microbiology laboratories where stored samples are used for research, there is a need to obtain the consent of the source, and a waiver from the authorised ethics committee if the source is not available for re-consent (37).
An important reason for making consent mandatory is that there are large cultural variations in the way people perceive of “biological waste,” such as sputum, urine and an excised tumour; these views can be different from a medicalised view (38). Article 22 of the European Union (EU) guidelines (https://wcd.coe.int/ViewDoc.jsp?id=977859) calls for an “independent evaluation” of the use of residual samples for research in laboratories in the absence of re-consent (39). In fact, Article 21 of the guidelines provides a “general rule” for the use of biological samples and states: “Research on biological materials should only be undertaken if it is within the scope of the consent given by the person concerned. The person concerned may place restrictions on the use of his or her biological materials.” (39) In this context, it is important that researchers in diagnostic laboratories recognise the need to formulate their research and subject it to the same standards of ethical scrutiny as other researchers. This might well be the case already, but undoubtedly there is potential for “opportunistic” research on stored samples in the absence of consent. The use of “residual” samples raises several issues. Why are the samples retained after primary analyses are completed? Are laboratories given clear guidelines on how long they are to store the samples and what they should use them for? Have they been given clear guidelines on the destruction of samples after primary analyses are completed?
In reality, the complexities of biobanking may result in situations in which research needs and the expectations or understandings of participants/donors diverge from each other. Informed consent acts as a safeguard for the organisation housing the biobank and the researchers involved, and thus becomes a “convenient, manageable solution for a complex, multi-dimensional problem” (9, 19).
The ICMR guidelines provide that informed consent should be obtained from donors for DNA and cell line banking. Among the other requirements are that donors should be informed about the purpose (shown to be nebulous at the time of the donation of the sample!), the conditions for the use of the sample by other researchers, how long the sample will be preserved and the costs involved in a researcher obtaining samples from the repository. These guidelines reflect the procedural focus and the operational and organisational aspects of the biobank. In case there is a possibility of commercial prospects, the guidelines emphasise the need for “appropriate benefit-sharing agreements” signed by the donor, sample collector and “repository in charge.” How these provisions are to be implemented and monitored is not specified. Community consultations are encouraged before the collection of samples with group consent, which is to be obtained before individual consent (15).
Withdrawal of consent/destruction of samples
While consent for storage and future use is an area of interest for the investigator, the donor’s right to destruction of the sample and discontinuation of participation in the research cannot be ignored. In an analysis of consent across different studies on biobanked samples, Hoyer suggests that to build up trust between donors and the organisation storing their tissues/data and to respect the contribution of the donor, all concerns relating to retaining samples should be taken seriously and the donor’s interests should be of paramount importance while framing the rules of storage, reuse and destruction (19). An individual may want to withdraw because of personal factors such as loss of trust in the researcher, institutional factors such as a change in the management, shutting down of the institution, or societal factors such as a public outcry against unethical practices revealed by the media. In the published literature, medical scientists suggest that once a person has given consent when providing a sample, he/she should not ask for its destruction subsequently (9), and that giving consent actually hands over control to the researcher to decide on the future use or destruction of the sample (30).
In its current guidelines, the ICMR recognises the right of an individual to withdraw from a study, but it does not indicate how this could be translated into practice and monitored. It states that “the sample collector must clearly inform every donor that he reserves the right to order destruction of his sample from the repository at any time.” Later in the same document, it is stated that “participants have the right to withdraw at any time, …..this does not apply to anonymised samples” (15). If anonymisation is the process used to maintain confidentiality, then the option of withdrawing the sample or ordering its destruction could well be lost with it.
Another thing that guidelines and the literature reviewed do not touch upon is the question of seeking re-consent for the storage and use of the tissue or sample of a child once the child attains adulthood and the legal age of consent.
A recommendation emerging from public deliberations in Australia suggested that biobank guidelines should include details about the destruction of samples and data on the completion of research, in a manner that protects privacy, is consistent with consent and is transparent to participants and the general public (40). This is clearly an important area since it deals with the responsibility of institutions in the governance of biobanks. The recommendation also emphasises the close link between ethical issues, on the one hand, and governance, on the other.
Confidentiality and anonymisation
One way of ensuring the individual’s right to privacy and upholding the principle of confidentiality is to minimise the connection between the identifiers and the stored sample or medical data, ie to delink the person from his/her biological material. The various ways of storing samples/data with varying degrees of identification are shown in Table 2.
Table 2 Methods of linking or delinking data/samples with their identifiers
| Identifiable | The identity of the person is directly attached or linked to the sample/data. |
| Traceable / coded or linked (15) | A code is attached to the sample/data and the correspondence between the code and the identity is physically separated. Only a few people are aware of the connection. |
| Encrypted | The code is transformed into several characters by a third party. The third party will be required to trace the individual identity. (This method was in use in Iceland’s deCODE biobank.) |
| Anonymised or unlinked (15) | The link between the samples/data and the individual identity is irreversibly cut. Therefore, they lack identifiers. |
| Anonymous or unidentified (15) | The sample/data were without any identifiers from the start. |
However, it can be argued that genetic identifiers can never be removed from any biological sample and hence, an individual can always be traced. Moreover, in biobanking research, the potential for long-term use depends on the ability to connect medical data with the sample over time. Therefore, as seen with the UK Biobank, it is necessary to maintain links with the contributor to update medical information and information on lifestyle (30).
It is also clear that the more sophisticated the system of coding, the more difficult it is to link the individual and the sample (9). What follows from this system of protecting identity is the individual’s loss of control over the sample being destroyed, or uncertainty over whether he/she will be informed about future research and the findings of the research, or whether he/she will receive a share of the benefits of the research (9, 13).
It appears from several studies that the level of concern over confidentiality and privacy varies across countries (10, 13, 28, 30, 40). This may be linked to the trust that contributors have in their governments and their healthcare systems. We do not know the views of participants in India and it is likely that perceptions will vary and become subject to change as individuals begin to question the potential uses and abuse of their biological samples.
The issue of anonymity is important in that the present regulations in India allow for the secondary use of a sample if provisions for ensuring the anonymity of the sample are made clear(15). In addition, anonymisation frees the researcher from the obligation of communicating the results to the donor. In cord blood and stem cell research, confidentiality is maintained, but the option of traceability of the participant in a contingency situation is kept open as “the donor might need to be contacted in future” (41).
Biobanking studies are associated with certain additional challenges in the area of maintaining confidentiality and anonymity. These include challenges arising from the large numbers handled in biobanks, the possibility of multiple investigators handling datasets, the transfer of samples, and changing investigators as samples are stored for long periods of time. However, advancements in the field of information technology may offer solutions, as the experience of various large biobanks has shown (9, 42). The keys to success are making budgetary provisions for these solutions and a system of oversight.
Several ethical issues arise following the collection of the sample and during the research itself. These are discussed below.
Storage of samples
Authors such as Scholtes et al have expressed concerns over the storage of samples over a period of time for repeated measurements, the main concern being the reproducibility and reliability of the stored assays (16). Institutions have a central role in ensuring the proper storage of samples so that the stated aims of the proposed research can be achieved. A case in point is the national guidelines for stem cell research, which give a clear idea of the conditions under which samples should be stored (41). In India, the Transplantation of Human Organs Act, 1994, together with its amendments in 2009 and 2011, regulates the removal, storage and transplantation not only of human organs, but tissues such as bone, skin and heart valves as well (43). It is our view that these regulations need to be incorporated into the ethical guidelines for biobank-related research. This is important since the onus of ensuring that adequate infrastructure and funding are available for the optimal storage of samples is placed on the institution. In the absence of such regulations, there is a likelihood that samples may be inadequately preserved, and consequently, less use would be made of samples than expected, the gains from research would be less than originally projected, and there would be an erosion of public trust.
Secondary or extended uses of stored samples
Another issue that arises in the case of a stored sample is the secondary or extended uses to which it might be put and the ethical issues relating to re-consent.The Norwegian Biobanks Act, 2003 states that re-consent or renewed “voluntary, explicit and informed” consent is required from the participants whenever a new/specific/altered/extended research study is being designed, using stored samples or data. When re-consent is impossible to obtain, the Norwegian Ministry of Health may make an exception (17). A number of the biobanks use broad consent to avoid obtaining re-consent. However, this broad initial consent denies contributors the chance to be a part of what is referred to as “upstream” decision-making (44).
The ICMR guidelines make no mention of re-consent as a requirement, saying only that “for secondary use of samples, the original consent shall not be transgressed…” (15). Subsequently, they state that requests for secondary use shall be examined by the institutional ethics committees. However, the ethics committee can act only when researchers refer the issue of re-consent to it. An important task before the ethics committees is to develop clear and unambiguous guidelines for researchers with regard to re-consent. Having said this, it is almost impossible to envisage all future scenarios. Researchers must be encouraged to approach the ethics committee whenever they are in doubt about the need for re-consent.
An alternative view is that participants have an equal right not to be contacted, that there is also something such as re-consent “fatigue” (9, 21) and that these two factors need to be considered at the time of obtaining consent. It is possible that individuals will prefer not to be informed about the future use of their samples, although this cannot be assumed. As Steinsbekk et al state, “respectful treatment of participants is not to ask them about everything but about things that matter” (45).
While in Norway, it is feasible to seek re-consent through the postal service, phone service and the local media and to provide information on the study through the research centre’s website, it would hardly be so in the Indian context. There are huge hurdles in the form of poor traceability of participants and the high costs of contacting them, and tackling these logistical issues would require commitment from the researcher, as well as time and finances. It is argued that insistence on obtaining re-consent would effectively kill research in this area. Interestingly, even in Norway, the latest deliberations suggest that instead of repeated seeking of consent, a “framework” of similar studies being considered can be provided to the participant for consent (45).
Thus, the issue of consent or of re-consent does not concern only the information that makes the research ethically sound, but also the context in which consent is obtained and the motives of the participants (13). This is particularly true in collectivist countries, such as India, where decision-making may not be autonomous (at an individual level) but involves members of the family and, possibly, of the community.
Findings of participants and informing participants
There has been a debate on the feedback given to participants in research. While some hold that no feedback should be given at all, others are of the view that feedback should be given under specific conditions, such as if the test result has clinical relevance and if there is a known therapy/intervention which is effective (46). The UK Biobank, for example, states clearly in its framework that “participants will receive no feedback at all, since the findings will all be in an aggregate form, with no release of individual data” (13). However, the findings of the basic assessment, ie blood pressure, body mass index (BMI) and other physical parameters, will be made known to the participants and, if they wish, to their general practitioner, at the time of their enrolment only (13). There is also the risk of “informational harm”, which can occur if participants are provided feedback when they are not prepared or cannot do anything with the information they receive; this can be distressing, at the very least (13). It may be a good approach to inform subjects/patients at the time of sample collection that novel information, beneficial or non-beneficial, may emerge once their sample has been studied and to ask them what must be done with this information (16). Participants may also choose not to be contacted about their results (9, 19). Another alternative is to give participants the option of receiving an aggregate report of all the results of the study through a newsletter, which, however, does not reveal individual results. This becomes a shared benefit for the community (17). In this era of social media and electronic access to information, biobanks are attempting to improve their information technology capacities and use this to disclose the results of studies both in the aggregated and individual forms (47).
The ICMR guidelines do not make any mention of providing results to participants.While this may be justified because of the logistical issues faced in India and the possible dangers of breaking confidentiality while reversing the encrypting process, it cannot perhaps be overlooked when certain baseline results which have a clear therapeutic implication for the participant, eg high blood pressure and diabetes, are withheld. In a resource-poor setting where access to routine investigations is low and it is difficult for the general public to afford investigations, the ethical issue of withholding findings from the treating physician or the individual is something that must be considered. No guidelines can address this unless they also deal with the practical implementation of the recommendations in this connection. It also highlights the need for independent monitoring.
Transfer of samples and data
It is unlikely that the host institution where a biobank is located will be in a position to perform the entire range of tests on biological samples “ín-house”. Also, in genomic research, among other types of research, large datasets are often required to advance science (20, 22) and therefore, laboratories with high throughput capability are needed. In these circumstances, it may be necessary to transfer samples to other laboratories, and share datasets (21, 12). Article 18 of the UNESCO Declaration also aims to promote and regulate the cross-border sharing of and access to genetic data and biological samples for the purpose of international cooperation and scientific advancement (8). The groups to whom transfers are made vary from academic collaborators, not-for-profit research and development foundations, government agencies to business and industry (12). Certain studies have shown that the majority of participants in biobank-related research favour granting access to DNA databases to medical personnel and academic and research scientists, and not to pharmaceutical industry, despite the latter’s role in developing drugs (2). Given that individuals often participate in biobank-related research on the basis of their trust in the host institution (13, 29, 30), it is advisable to identify partner institutions on the basis of shared values as far as possible. When this is not possible, the clearly stated use of transferred samples, returning residual samples, reporting requirements, and delinking clinical and other data from the transferred sample are particularly important to ensure continued trust in the process.
Different countries have different legislations to protect data and privacy. For example, Norway requires re-consent for such transfers of samples and biological data (45). In India, material transfer agreements (MTAs) are already a requirement if samples need to be transferred abroad and need regulatory approval (icmr.nic.in/guide/mta.doc). There is a need to extend this to the area of biobank-related research and the institutions involved within the country, with a clear delineation of roles and responsibilities, since the issues of confidentiality, commercialisation, etc are essentially the same as in the case of the transfer of samples abroad. MTAs are also a good way of tracing the biospecimens and data, as well as ensuring transparency and accountability (48). It would be ideal for the institutional ethics committee, or more appropriately, the biorepository ethics committee (15) to develop clear guidelines and mandatory clearances for investigators in their institutions.
To be able to sustain research and translate the findings into health benefits, ie therapies and interventions, financial resources are required (2, 18). These generally become available through collaboration with other stakeholders. Thus, ethical questions related to commercialisation, the ownership of tissues and results, the sharing of benefits and the assessment of outcomes arise (2, 10, 49).
Commercialisation
This is a serious and contentious issue worldwide. It is argued that the involvement of commercial and pharmaceutical companies is essential in making wider health benefits a reality and hence, they should be entitled to seek rights over the products of the work that they support (2, 3). An alternative view affirms the importance of open and free access to the new knowledge created through what is essentially public contribution, and the “injustice of using a gift to make profit” is perceived as “disrespect to people” (2). It is also argued that commercialisation is necessary for the maintenance of the facilities of biobanks and that there are a number of private companies showing an interest in storing samples or supporting their storage (16).
Given the attractiveness of biobanking, the commoditisation of human biological material has also become an ethical concern (44). This is an echo of the concern over the continued sale of human organs in India, despite legislation banning it. The UK government uses its various legislations – the Human Tissue Act, 2004, the Human Organ Transplants Act, 1989 and the Anatomy Act 1984 – to regulate the use of human tissues for research (50).
Studies carried out in Norway, the UK, Australia and New Zealand indicate that while participants appeared to trust individual researchers, the specific institutions housing the biobank and the government systems regulating the biobanks, there was scepticism about and fear and distrust of “for-profit organisations”, industry and commercial entities (2, 17). The question that then arises is whether in a developing country, the health ministry and/or the government research authorities can be mandated to set aside funds to support well-administered biobanks so that individual institutions will not be compelled to seek funds from private/commercial players for the sustained management of the biobank.
Ownership of samples and results
An issue related closely to commercialisation is that of ownership and privacy of the tissue and data. On one side are the intellectual property rights of the investigators and on the other, pressure for an “open exchange/sharing” of datasets (2, 9). There is also the question of the donor’s uncertainty about the ownership of his/her biological material (as was the case with John Moore and Henrietta Lacks, two well-known cases) and the extent to which the right to ownership is handed over while signing the consent forms (19, 34). Researchers suggest that at the time of entering into partnership with commercial bodies/private companies for the management of the biobank or in support of the research and its translation, it is important to define who owns the tissue (2). Since there are clearly multiple stakeholders in a biobank – the donors, investigators, funding agencies, institution housing the biosamples and ethics review committee – it has been proposed that the institution of the biobank should hold “custodianship” for the use of the resource, and that the custodian of the samples should fulfil numerous responsibilities. These include strict adherence to ethics and regulations, commitment to ensuring the stated scientific outcomes and translating the scientific outcomes into broader health benefits (13, 22). However, vesting the ownership of the biobank’s resources in the biobank does not mean that contributors cannot negotiate their rights, as is upheld in the Australian guidelines (48). In the UK Biobank, the data and the samples are held in “custodianship” or “stewardship” by the research governance bodies (2). There is also the feeling that population databases should be “publicly owned”, like public charities (2). The Indian equivalent is a public trust or charitable society (a non-profit, non-governmental entity). Community-based studies in societies which are more focused on the community than the individual stress the importance of collective determination and not singularly individual autonomy and individual rights of ownership and gain (29).
Sharing of benefits
Commercialisation also raises the issue of the sharing of benefits. Article 19 of the UNESCO Declaration (2003) states that there are multiple forms of benefit that include special assistance to persons/groups that participated in the research, access to medical care, provision of new diagnostics and facilities for new treatments or drugs stemming from the research, support for health services and capacity-building facilities for research purposes(8). Therefore, benefit goes beyond monetary or economic gain (2).
An additional issue is whether the sharing of benefits should be seen in terms of the individual donor/participant or the wider community to which the donor belongs. In Australia, a public deliberation study (40) on the policies of biobanking reinforced the view that it would be best to pursue the policy in place for benefit-sharing with the community by way of using royalties and levies on profits to finance health infrastructure, etc. Benefit-sharing was clearly not seen in terms of benefits for individuals, such as reduced medical bills for treatment or medication. Denying contributors some community benefit would be a betrayal of the trust they have in the institution or the research (Campbell, 2007).
The majority view across various sociological studies is that participants in biobank-related research should not benefit directly because it could coerce people into participating in such research, and it could have an unduly large impact on vulnerable groups. It is also suggested that allowing participants to benefit directly would not be congruent with the concepts of altruism, the common good and trust, which should be the motives for participation. A philosophical point to consider is whether the stakeholders (researchers, governments, financiers, etc) involved in biobank-related research should be driven by anything less than altruism when this is what is expected of the participants. Another pertinent issue is whether it is ethically correct in a resource-poor setting like India to expect people to participate solely out of altruistic motives, when health needs are so high and healthcare facilities so poor.
The ICMR guidelines on DNA/cell line banking are explicit about benefit-sharing, stating that “if any commercial use of the samples in the repository is made, appropriate written benefit-sharing agreements, jointly signed by the donor, sample collector and the repository in-charge” should be made (15). What is not clear is how these benefit-sharing guidelines should be translated into practice, particularly in resource-poor settings. In addition, the guidelines are not clear on how benefits accrue to a participant if the samples are anonymised or if the participant is not traceable. Again, there is a need to explicitly state the role/responsibilities of the institutional ethics review committee in such situations. It would also be interesting to know if there are any examples of these guidelines being used effectively to negotiate the sharing of benefits in India or similar resource-poor countries. It becomes difficult for regulatory bodies, such as institutional ethics committees, to make risk-benefit calculations, given that at the time of review, the outcomes, prospect of commercialisation and possibility of benefits are unclear.
Assessment of outcomes
We started out discussing how the “people” and their health needs are central to a biobank, then considered the biobanking research process and the extent to which it focuses on people. In this section, we deal with the assessment of the outcomes of the research.
The ethical aspects of the outcomes of research are often overlooked. Who assesses the value of the outcome? How is it assessed? Has the research achieved its stated aims? Should the outcome be evaluated in terms of the returns on investment and generation of revenue through diagnostic tests and new medication, or in terms of the research having achieved its stated aims and making it possible to deliver better health to people? This question is especially relevant in the context of developing countries such as India. There is a sense that private investment in biobank-related research would target “profitable diseases” (19) rather than address the health concerns of the poor majority. In addition, when research findings are in the hands of governments, insurance companies or employers, there is a possibility that they may be used exploitatively and the marginalisation of minorities may be a consequence (30). Certain countries have passed legislation against the use of genetic testing to discriminate against individuals in the contexts of insurance and employment.
Openness about the outcomes of research is critical in ensuring and sustaining public trust in the biobanking process and in dispelling doubts about the collection of samples. Again, it is the governance body of the biobank which, as a neutral objective body, can provide the ethical oversight in this matter. If the governance body is to fulfil this role, its composition is of vital importance. While finding “safe hands” may be difficult, the diversity of representation in the governance body would be helpful (30). As for the UK Biobank, there is an Ethics and Governance Council and in the case of Generation Scotland, there is an advisory board which has additional oversight and regulatory functions over and above the standard ethical review function. Termed “regulation plus” (2) by Haddow et al, this strengthens the accountability of all the stakeholders involved.
Conclusion
In summary, there are multiple stakeholders in biobanking research. The current regulations in biobanking research reflect the “subordinate” role of the individual providing the sample. While ethical regulations/guidelines in research have evolved to protect the interest of the individual, the reality is that once the contribution is made, the connection between the individual and the biobank is largely lost. At present, there is not much ethical discourse on the responsibility of institutions to ensure optimal storage of samples and transparency in their use. Finally, there is a need to ensure monitoring of research outcomes and the translation of the research vis-a-vis the stated aims; this is critical for the maintenance of public trust in research and the perception that research is relevant for the public. From most research publications on the ethics of biobanking, it is evident that a robust system of ethics and governance of biobanks is a critical factor, without which biobanking “may be doomed to fail” (10). It is also clear that winning the trust of the public is the key to functioning in an ethical manner.
While the UNESCO draft declaration of 2003 accords a “special status” to genetic data, its implementation is complicated by the fact that not all data that are used for research may be genetic. Hence, non-genetic data are left out of the purview of the rules in the declaration. Further, various researchers have shown that there are many similarities between genetic and non-genetic health data, and that all data contained in a biobank, whether genetic, clinical, genealogical, lifestyle-related or environmental, should be considered “special.” In this way, all data would be covered by the biobank’s system of ethics and governance.
The conventional bioethical understanding of biobanking – the medical and the knowledge enhancement models – is limiting and needs to be explored in the psycho-socio-cultural context of India. It is important to base policies and regulations on the issues and problems experienced and perceived in a particular setting or context, rather than on imagined problems or problems relevant to a different context or setting (44). Universally, it has been observed that the present focus is on the researcher and safeguarding research interests, using an “analytical moral philosophy” of ethics, while losing sight of the social, cultural and political aspects involved (12). Hence, there is a greater need to understand public perceptions and the readiness of the people to participate in biobanking research. It is necessary to commit to a deeper, wider engagement with the community to ensure equity, transparency and trust.
References
- Ashcroft R. The ethics of reusing archived tissue for research. Neuropathol Appl Neurobiol.2000 Oct;26(5):408-11.
- Haddow G, Laurie G, Cunningham-Burley S, Hunter KG. Tackling community concerns about commercialization and genetic research: a modest interdisciplinary proposal. Soc Sci Med. 2007 Jan;64(2):272-82.
- Pullman D, Latus A. Clinical trials, genetic add-ons, and the question of benefit-sharing. Lancet. 2003 Jul 19;362(9379):242-4.
- Human Genome Organisation Ethics Committee. [Internet]. Statement on benefit-sharing. April 9 2000. [Cited 2013 Feb 23]. Available from: http://www.hugo-international.org/img/benefit_sharing_2000.pdf
- Wallace HM. The development of UKBiobank: excluding scientific controversy from ethical debate. Critical Public Health. 2005; 15(4):323-33.
- Walmsley HL. Mad scientists bend the frame of biobank governance in British Columbia. Journal of Public Deliberation. 2009; 5(1) Article 6. Available from: http://www.publicdeliberation.net/jpd/vol5/iss1/art6
- Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med (Berl). 1996 June;74(6):297-312.
- UNESCO, Bioethics Committee. [Internet]. International Declaration on Human Genetic Data. 2003. [Cited 2013 Feb 23. Available from: http://www.unesco.org/new/en/social-and-human-sciences/themes/bioethics/human-genetic-data/
- Cambon-Thomsen A. The social and ethical issues of post-genomic human biobanks. Nat Rev Genet. 2004 Nov;5(11):866-73.
- Boverberg JA. Towards an international system of ethics and governance of biobanks: A ‘special status’ for genetic data? Crit Public Health. 2005;15(4):369-83. doi: 10.1080/09581590500523400
- Mitchell R. National biobanks: clinical labor, risk production and the creation of biovalue. Sci Technol Human Values. 2010 May 1;35(3):330-55. doi:-10.1177/0162243909340267
- Corrigan OP, Williams-Jones B. Pharmacogenetics: the bioethical problem of DNA investment banking. Stud Hist Philos Biol Biomed Sci. 2006;37:550-65.
- Campbell AV. The ethical challenges of genetic databases: safeguarding altruism and trust. King’s Law J. 2007;18:227-46.
- Sheehan M. Can broad consent be informed consent? Public Health Ethics. 2011 November;4(3):226-35. doi: 10.1093/phe/phr020
- Indian Council of Medical Research. Ethical guidelines for biomedical research on human participants [Internet]. New Delhi: ICMR; 2006 November [cited 2014 Jan 14]. 111 p. Available from: http://www.icmr.nic.in/ethical_guidelines.pdf
- Scholtes VPW, de Vries JPPM, de Kleijn DPV, Moll FL, de Borst GJ, Pasterkamp G. Biobanking in atherosclerotic disease, opportunities and pitfalls. Curr Cardiol Rev. 2011 Feb;7(1):9-14. doi: 10.2174/157340311795677707.
- Skolbekken JA, Ursin LO, Solberg B, Christensen E, Ytterhus B. Not worth the paper it’s written on? Informed consent and biobank research in a Norwegian context. Crit Public Health. 2005;15(4):335-47.
- Palsson G, HarDardottir KE. For whom the cell tolls: debates about biomedicine. Current Anthropology. 2002;43(2):271-301.
- Hoyer K. The ethics of research biobanking: a critical review of the literature. Biotechnol and Genet Eng Rev. 2008;25:429-52.
- Zika E, Paci D, Braun A, Rijkers-Defrasne S, Deschenes M, Fortier I, Laage-Hellman J, Scerri CA, Ibarreta D. A European survey on biobanks: trends and issues. Public Health Genomics. 2011;14(2):96-103.
- Knoppers BM, Laberge CM. Research and stored tissues. Persons as sources, samples as persons? JAMA. 1995 Dec 13;274(22):1806-07.
- Organisation for Economic Cooperation and Development (OECD) [Internet]: OECD Guidelines on human biobanks and genetic research databases. 2010 [Cited 2013 Feb 23] Available from: http://www.oecd.org/science/biotech/44054609.pdf
- Austin MA, Harding S, McElroy C. Genebanks: a comparison of eight proposed international databases. Community Genet. 2003;6(1):37-45.
- Peterson A. Biobanks: Challenges for ‘ethics’. Crit Public Health. 2005a;15(4):303-10.
- Sgaier SK, Jha P, Mony P, Kurpad A, Lakshmi V, Ganguly NK. Public health. Biobanks in Ndeveloping countries: needs and feasibility. Science. 2007 Nov 16;318(5853):1074-75.
- Shaw D, Elger BS. The relevance of relevance in research. Swiss Med Wkly. 2013 May 7;143:w13792. doi: 10.4414/smw.2013.13792.
- Sumner J. Public attitudes to biobanks and related ethics and governance issues. Final Report. Edinburgh: UK Biobank Ethics and Governance Council; 2007.
- Stranger M, Chalmers D, Nicol D. Capital, trust and consultation: databanks and regulation in Australia. Crit Public Health. 2005;15(4):349-58.
- Scott A, Phillips H, Moore A, Du Plessis R. Ethics in practice: conversations about biobanks. Crit Public Health. 2005 Dec;15(4):359-68.
- Levitt M, Weldon S. A well placed trust?: Public perceptions of the governance of DNA databases. Crit Public Health. 2005 Dec;15(4):311-21. doi: 10.1080/09581590500523186
- Shah JY, Phadtare A, Rajgor D, Vaghasia M, Pradhan S, Zelko H, Pietrobon R. What leads Indians to participate in clinical trials? A meta-analysis of qualitative studies. PLoS One. 2010 May 20;5(5):e10730. doi:10.1371/journal.pone.0010730
- DeCosta A, D’Souza N, Krishnan S, Chhabra MS, Shihaam I, Goswami K. Community-based trials and informed consent in rural north India. J Med Ethics. 2004 June;30(3):318-23.
- Wendler D, Emanuel E. The debate over research on stored biological samples: what do sources think? Arch Intern Med. 2002 Jul 8;162(13):1457-62.
- Skloot R. The immortal life of Henrietta Lacks. 2nd ed. London: Pan Books; 2011.
- Kaye J. Abandoning informed consent: The case of genetic research in population collections. In: Tutton R, Corrigan O, editors. Genetic databases: socio-ethical issues in the collection and use of DNA. London: Routledge; 2004:117-38.
- Godard B, Schmidtke J, Cassiman JJ, Ayme S. Data storage and DNA banking for biomedical research: informed consent, confidentiality, quality issues, ownership, return of benefits. A professional perspective. Eur J Hum Genet. 2003 Dec; 11 (Suppl 2):S88-S122.
- Desikan P, Chakrabarti A, Muthuswamy V. Ethical issues in microbiology. Indian J Med Microbiol. 2011 Oct-Dec; 29(4):327-30. doi: 10.4103/0255-0857.90154
- Jenkins GL, Sugarman J. The importance of cultural considerations in the promotion of ethical research with human biologic material. J Lab Clin Med. 2005 Mar;145(3):118-24.
- Council of Europe. Recommendation Rec (2006) 4 of the Committee of Ministers to member states on research on biological materials of human origin [Internet]. 2006 Mar15[cited 2014 Jan 14]. Available from: http://wcd.coe.int/ViewDoc.jsp?id=977859
- Molster C, Maxwell S, Youngs L, Potts A, Kyne G, Hope F, Dawkins H, O’Leary P. An Australian approach to the policy translation of deliberated citizen perspectives on biobanking. Public Health Genomics. 2012;15(2):82-91. doi: 10.1159/000334104
- Indian Council of Medical Research (ICMR), Department of Human Research & Department of Biotechnology. Guidelines for stem cell research (Draft). New Delhi: ICMR; 2012 [cited 2014 Jan 14]. Available from: http://icmr.nic.in/stem_cell_guidelines.pdf
- Nietfeld JJ, Sugarman J, Litton JE. The Bio-PIN: a concept to improve biobanking. Nat Rev Cancer. 2011 Apr;11(4):303-8. doi: 10.1038/nrc3022.
- Government of India. The Transplantation of Human Organs Act, 1994. [cited 2013 June19] Available from: http://www.bareactsonline.com/pdfs/2010/THE%20TRANSPLANTATION%20OF%20HUMAN%20ORGANS%20(AMENDMENT).pdf
- Hoyer K, Tutton R. ‘Ethics was here’: studying the language games of ethics in the case of the UK biobank. Crit Public Health. 2005 Dec;15(4):385-97.
- Steinsbekk KS, Solberg B. Biobanks – when is re-consent necessary? Public Health Ethics. 2011;4(3):236-50.
- Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, Cho MK, Christman MF, Green RC, Hall R, Illes J, Keane M, Knoppers BM, Koenig BA, Kohane IS, Leroy B, Maschke KJ, McGeveran W, Ossorio P, Parker LS, Petersen GM, Richardson HS, Scott JA, Terry SF, Wilfond BS, Wolf WA. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet Med. 2012 Apr;14(4):361-84. doi: 10.1038/gim.2012.23.
- Kozlakidis Z, Cason RJS, Mant C, Cason J. Legal and ethical issues in biobanking. In: Ghista DN, Editor. Biomedical science, engineering and technology. InTech;2011:761-78. doi: 10.5772/1020
- National Health and Medical Research Council (Australia). Biobanks information paper [Internet]. Canberra: Australian Government; 2010 Feb[cited 2014 Jan 14] Available from: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/e110_biobanks_information_paper.pdf
- Knoppers BM. Population genetics and benefit sharing. Community Genet. 2000;3(4):212-14.
- McHale JV. ‘Appropriate consent’ and use of human material for research purposes: thecompetent adult. Clinical Ethics. 2006 Dec 1;1(4):195-99.