DISCUSSION ETHICS OF ETHICS COMMITTEES
Competence of ethics committees in patient protection in clinical research
Pratibha Nadig, Medha Joshi, Aradhana Uthappa
DOI: https://doi.org/10.20529/IJME.2011.061
Abstract
Research Ethics Committees (RECs) are responsible for the protection of patients’ rights and wellbeing. In this paper, we describe the findings of a survey of ethics committee members in a south Indian state. 29 members of 11 RECs responded to a questionnaire of 56 questions on their knowledge of and attitudes towards ethics review and the practices of the RECs to which they belonged.
Introduction
Research Ethics Committees (RECs) play a critical role in the conduct of good research. They are responsible for the protection of patients’ rights and wellbeing. The Declaration of Helsinki (1) and the Good Clinical Practice (GCP) guidelines of the International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use (2) have set international standards for ethics review of clinical research.
In India, clinical trials are governed by Schedule Y in the Drugs and Cosmetics Act (3). Schedule Y requires that the study protocol be reviewed and approved by an REC, following the Indian Council of Medical Research’s (ICMR’s) ethical guidelines for biomedical research (4). The ICMR guidelines lay down various requirements for RECs, including their composition and the review and decision making process. The REC must include members from scientific as well as non-scientific backgrounds. It must conduct a thorough ethics review, be independent in taking decisions, and have written procedures for its functioning.
In 2003, the ICMR with the World Health Organization conducted a survey on 223 institutional ethics committees in India (5) which found that many committees did not meet regulatory requirements in terms of composition and function. Since then, no reports have been published on this subject. In this paper, we describe the findings of a survey of ethics committee members from three cities in a south Indian state.
Methods
A questionnaire was prepared based on the ICMR-WHO survey (5) with the addition of questions in order to address three domains: respondents’ knowledge of ethical guidelines, their attitude towards ethics review, and the practices followed by the REC to which they belonged. The survey was carried out from November 2008 to December 2009 after obtaining ethics committee approval.
The questionnaire was pre-tested on subject experts and members of RECs. The revised questionnaire had 56 questions: 12 addressed respondents’ knowledge of ethics review, 17 enquired about their attitudes to review, and 27 concerned the practices of the RECs to which they belonged. Knowledge and practice were assessed through a mix of open ended, multiple choice and true/false questions. Questions on attitudes used a 5-point Likert scale.
Those Research Ethics Committees involved in the review of sponsored clinical trial protocols and which had an experience of reviewing at least 10 protocols were identified and included. The names of the committees were obtained through our contacts with sponsors and investigators. Four cities were identified representing the North, South, East and West parts of the state.
The questionnaire was sent to members of 20 ethics committees after verbal permission was taken from the committees’ chairpersons. The authors met the chairpersons personally to brief them about the study, after which the questionnaires were sent by e-mail or courier, or hand delivered. An accompanying covering letter stated that participation was voluntary; the study was for academic purposes, and confidentiality of participants and committees would be strictly maintained.
In all the cases the questionnaire was routed through the chairpersons. If there was no response after 30 days, the chairpersons were sent a reminder. If there was no response 30 days after this reminder, the authors visited personally to collect the responses from individual members after fixing up an appointment. One response was received online. The remaining responses were either collected personally or, in the case of committees outside Bangalore, received by courier. Responses from one committee were received only after one of the authors made a presentation to committee members on the study. The responses from other cities were received by courier.
Analysis
Descriptive statistics were used. For the practice- and knowledge-related questions, the frequency of correct (as defined by the ICMR guidelines) responses for each question was calculated and expressed as a percentage of the total and 95% confidence interval (CI). Likert ul analysis was carried out for the questions on attitudes.
Results
Of the 20 ethics committees contacted, responses were obtained from members of 11 committees (response rate 55%) representing three cities of the state. Nine committees were institutional RECs and two were independent committees. Of the nine institutional committees, two were private medical colleges. The remaining were from private hospitals and research institutions conducting clinical trials. The year of establishment of the committees ranged from 1999 to 2007. A total of 29 members from 11 committees completed the survey.
Profile of respondents
Out of 29 respondents, 15 were men and 14 were women. Their educational background is given in Figure 1.
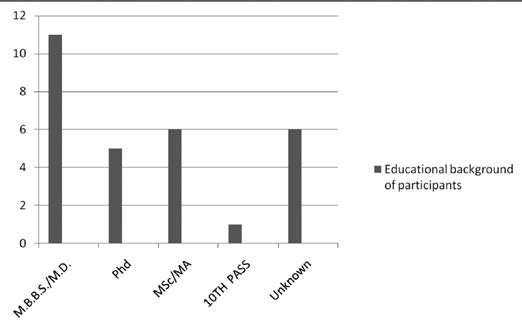
Seven respondents were the member secretaries of their REC and one was the chairperson. Two respondents were legal professionals, five were social workers or theologians, nine belonged to the layperson category, three were basic scientists, and one was a clinician. The background of one respondent is unknown.
REC composition and procedures
Membership and quorum: All the respondents reported that their committees contained a minimum of seven members and required a quorum for decisions. However, only 21% [6; 10-39] members were aware of how many people were necessary for a quorum.
Independence: All the respondents stated that their committees were independent in functioning and followed written standard operating procedures. 76% [22;58-88] reported that subject experts were invited when required. 24% [7; 12-42] stated that internal audits were conducted in their committees.
Honorarium: 97% [28; 83-99] respondents said they were paid some honorarium for participating in the REC. One person said their committee did not receive any honorarium.
Meetings: The frequency of meetings ranged from once a week to once in two months. 55% [16; 37-72] respondents said they met once a month, 28% [8; 15-46] met once a week, and 10% [3;4-27 ] met once in two months.7% [2;2-22] respondents reported that the committee met as and when required.
Guidelines: All the respondents reported that their REC followed the ICMR guidelines (2006) while reviewing protocols. 52% [15; 34-69] members also referred to ICH-GCP; 31% [9; 17-50] followed WHO GCP guidelines in addition to those of the ICMR,14% [4;6-31] also mentioned Schedule Y (2005). One member said they referred only to ICMR guidelines.
RECs’ review procedures
The number of research proposals discussed per meeting ranged from 1 to 20. 83% [24; 65-92] respondents stated that proposals were sent to them two weeks prior to the REC meeting.93% [27; 78-98] stated that the results of the discussion were communicated to the investigators within a week. All stated that the documents were archived for five years.
All the respondents stated that they reviewed the clinical trial protocol, informed consent form and case report forms. 28% [8; 15-46] stated that they also reviewed the translations of informed consent forms in various languages. 21% [6; 10-39] also reviewed the financial agreement between sponsor and researcher. All the respondents stated that they reviewed the study design in relation to its objectives and the informed consent process described in the protocols. 52% [15; 34-69] said they were provided with checklists for the review of clinical trial documents.
83% [24; 65-92] members said the decision to approve/reject the protocol was taken during the meeting with all members participating in the final decision making.
93% [27; 78-98] respondents said that periodic ethics review of ongoing trials was conducted. 28% [8; 15-46] respondents representing 3 RECs reported that on site monitoring was conducted. 72% [21; 54-85] respondents stated that the REC received a copy of the report at the end of the clinical trial. 52% [15,34-69] the respondents had received formal training in GCP.
There were 16 questions out of the total 27 practice questions pertaining to the elements of protocol reviewed. Each right answer was given a score of one. The mean score out of 16 was 12.96 [11-14].
Respondents’ attitude towards their roles and responsibilities
Responses to 17 questions addressing attitudes were given on a Likert scale.
76% [22; 58-88] of the respondents strongly disagreed with the statement that trials may start before REC approval in order to save time. 69% [20; 51-83] agreed or strongly agreed that the key focus of ethics committee approval is patient protection. All also strongly disagreed with the statement that ongoing trials need not be monitored by the REC. 91% [27; 78-98] of respondents strongly disagreed that the honorarium might improve their performance. 41% [12; 32-51] felt the need for training of members, 34% [10; 25-44] for regulations for ECs, and to define a limit on the number of protocols reviewed per meeting 28% [8; 20-38].
Respondents’ knowledge base
Knowledge of the respondents regarding regulatory guidelines, ethical principles, and clinical trial documents was assessed in 12 questions. More than 50% answered all the questions correctly. However, 69% [20; 51-83] were not aware of the different phases of clinical trials and 83% [24; 65 -92] could not name the regulatory body that approves the conduct of trials in India. The responses were also scored individually and the mean score out of 12 was 10.3[8-12].
Discussion
Based on the responses given by REC members in this survey, all the committees covered in this survey seem to function independently and with appropriate representation of persons with different qualifications as specified by the ICMR guidelines.
However, many members were unaware of the quorum requirement. Decisions taken by an ethics committee in the absence of quorum are not valid as per Schedule Y (3)
One third of committees reported conducting internal audits to ensure the quality of their procedures and function. This is an encouraging sign.
Onsite monitoring by RECs has been shown to prevent fraud and malpractice (6). In our survey, though many respondents reported that their RECs carried out periodic reviews of ongoing trials, only a few indicated that they carried out onsite monitoring.
The REC should examine the financial agreement between the investigator and the sponsor as any financial incentives can have ethical implications for the research. However, most committees did not review this document.
Though all respondents reported receiving the English version of the informed consent form, they did not review the back translations of the local language forms into English. In India, informed consent forms are prepared in many local languages and it is necessary to verify the translation of the informed consent form into the local languages and its back translation into English to ensure that all participants get the same information. This is a requirement as per Schedule Y but the RECs surveyed are apparently unaware of this requirement.
Training in GCP is meant to equip REC members to conduct effective ethics review. The need for training was apparently felt by only 12(41%) of the respondents which implies that they are not completely aware of their responsibilities
4 (14%) respondents were unsure of the documents required to be provided to participants. Though the letter of approval from the Drugs Controller General of India is an essential document to be submitted to the ethics committee, 5(17%) members could not name the regulatory body for clinical trials in India.
Further, though the quality of ethics review can be affected by the workload, many members did not feel the need to restrict the number of protocols to be reviewed per meeting.
It is also a matter of concern that 41% felt that REC approval posed a hurdle in the process of clinical trials. This suggests that the critical role of RECs in the review process is not understood by all members.
The first survey on RECs was conducted by ICMR-WHO in 2003. 1,200 questionnaires were mailed to medical institutions out of which 223 responded (response rate: 18.58 %). It was observed that REC members were appointed by lobbying; many committees did not include legal experts; standard operating procedures were not followed, and records were poorly kept (5). The ICMR conducted a survey of RECs of institutions conducting clinical trials funded by the ICMR in 2006-2007 (7).The response rate was 42.5%. 64% of the committees had standard operating procedures for review, 39% had members trained in bioethics and almost all had a multidisciplinary composition as per ICMR norms. Our study had a response rate of 55 %. 52% reported training in good clinical practice. All the RECs had written standard operating procedures and met requirements for the composition of the committee.
The findings of our survey suggest that there have been some improvements in the functioning of RECs in the past decade. However, our survey was based on a small sample, was restricted to a single state, and had a poor response rate. Our study should be viewed as the first step towards collecting more systematic information on the functioning of RECs in India.
We suggest that mandatory registration, accreditation and regular audits will provide such information, in addition to performing the function of regulating RECs.
Acknowledgements:
The authors are thankful to Vydehi Institute of Medical Sciences and Research Center and Bilcare Research Academy for their support.
References
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects [Internet]. Adopted by the WMA General Assembly, Helsinki, Finland, June 1964 and last amended at the 59th WMA Assembly, Seoul, October 2008. [cited 2011 Jun 13]. Available from: http://www.wma.net/en/30publications/10policies/b3/
- The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use [Homepage on the Internet]. [cited 2011 Jun 13]. Available from: http://www.ich.org
- Ministry of Health and Family Welfare. Drugs and Cosmetics (II Amendment) Rules, 2005. [cited 2011 Jun 13]. Available from: http://www.drugscontrol.org/Schedule_Y.pdf
- Indian Council of Medical Research. Ethical guidelines for biomedical research on human participants. New Delhi: ICMR; 2006.
- Kumar N. Bioethics activities in India under ICMR. Powerpoint presentation [Internet]. [cited 2011 Jun 13]. Available from: http://www.icmr.nic.in/bioethics/cc_biothics/presentations/haryana/activity.pdf
- Blunt J, Savulescu J, Watson AJ. Meeting the challenges facing research ethics committees: some practical suggestions. BMJ. 1998 Jan 3;316(7124):58-61.
- World Health Organization/Indian Council of Medical Research. Status of ethical review of ICMR funded projects in clinical research /clinical trials [Internet]. Summary report. 2006-2007. [cited 2011 Jun 13]. Available from: http://bw.businessworld.in/PDF_upload/ICMR.pdf
