THEME – ETHICAL AND LEGAL CHALLENGES OF VACCINES AND VACCINATION
An idea whose time has come: Compensation for vaccine-related injuries and death in India
Sarojini Nadimpally, Sneha Banerjee, Deepa Venkatachalam, Divya Bhagianadh
DOI: https://doi.org/10.20529/IJME.2017.023
Abstract
This paper emphasises the urgent need for a compensation policy for those affected by adverse events following immunisation in India. In the absence of such a mechanism in the country, people claim compensation by taking recourse to tort law and have to face the ensuing uncertainty and challenges with regard to the award of compensation. The paper argues that people should be provided compensation in the event of death and serious adverse events following compulsory immunisation, irrespective of whether there is a causal association between the adverse event and the vaccine, on the basis of no fault compensation.
Introduction
The Oxford English Dictionary defines “compensation” as “something, typically money, awarded to someone in recognition of loss, suffering or injury” (1). The obligation to compensate a person for injuries is grounded in human rights and the ethical principles of justice and fairness. According to WD Ross, reparative justice (sometimes used interchangeably with compensatory justice) requires that when we inflict an injury on others, we have a duty to apologise and repair the wrong done (2). Ross states that reparative action is morally indispensable, not only to repair the damage, but also to acknowledge the injured party as a moral agent worthy of respect and entitled to a confession of fault (2). Even when the argument in favour of reparative justice is accepted in principle, its actualisation is limited or fraught with complexities, as is evident from the existing compensation frameworks.
In the context of clinical research, for example, compensation frameworks mandate that if an untoward event occurs or a participant in a trial undergoes a serious adverse event (SAE)1, whether during or after the trial, medical treatment must be provided and adequate compensation ensured. Vaccines, which are generally administered on a mass scale to healthy people and mainly to children, often through the Universal Immunisation Programme (UIP), like other biological products and drugs, can give rise to adverse events following immunisation (AEFIs)2. However, these may be considered too statistically insignificant to warrant compensation. Globally, therefore, the issue of compensating people for harm or injury following the administration of vaccines remains a matter of debate, and only about 19 countries provide such compensation. Even where frameworks for compensation exist, in the case of AEFIs, their implementation differs across countries, with historical specificities and legal traditions shaping them.
This paper provides a brief overview of the existing mechanisms for compensation following the administration of vaccines in different countries. It asserts the need for compensation and recommends possible mechanisms founded on ethics and human rights for their implementation in India.
The nature of vaccines and the need for compensation
Vaccines are the only biological products that are given to people on a mass scale and are viewed as one of the most successful preventive measures against the infectious diseases they are meant to target. Just as many medical interventions can cause adverse effects, vaccination can lead to AEFIs, which can result in injury, hospitalisation and sometimes, death due to the vaccine itself. Death might take place due to some known or unknown side-effects of the vaccine, which may occur in a few or a large number of people. Death might also occur because the vaccine is not manufactured, stored, distributed or administered properly.
However, a frequently asked question is – which injuries and deaths can be designated as vaccine-related? How do we establish the causal associations between the AEFI and the vaccine? Is it important for there to be a causal relation for the payment of compensation? Or should it be paid irrespective of the causal assessment? (3). Also, how do we go about investigating an AEFI, and then documenting and publicising the findings?
Given this scenario, fixing accountability for the occurrence of AEFIs becomes a matter of debate and contestation. Who should be held responsible and who will provide the compensation – the vaccine manufacturer, the healthcare worker, the physician administering the vaccine or the State implementing the immunisation programme? In case compensation is not provided, what remedies are available for those suffering AEFIs?
More fundamentally, have any studies been carried out on the vaccine, and what are the benefits and risks of a specific vaccine? What is the actual burden of the disease for which the vaccine is being introduced and do other preventive measures exist? What is the system of monitoring? What kind of responsibility would it entail to treat/take care of any adverse events?
Global scenario
Almost 19 countries have instituted compensation mechanisms – whether through the courts or a compensation scheme payout – for individuals inadvertently injured by a vaccine programme or for death following vaccination. The timeline in Figure 1 indicates when different countries instituted vaccine-related compensation mechanisms. Germany and France initiated them in the 1960s, while in the USA, the Vaccine Injury Compensation Program (VICP) came into effect in 1988. The VICP is a federal “no-fault” system, designed to compensate individuals or the families of individuals who have been injured by covered childhood vaccines, whether administered in the private or public sector (4).The most recent compensation mechanisms were initiated in Slovenia and Hungary during 2004–05.Only two countries from Asia, ie Japan and Taiwan, have provisions for vaccine related compensation.
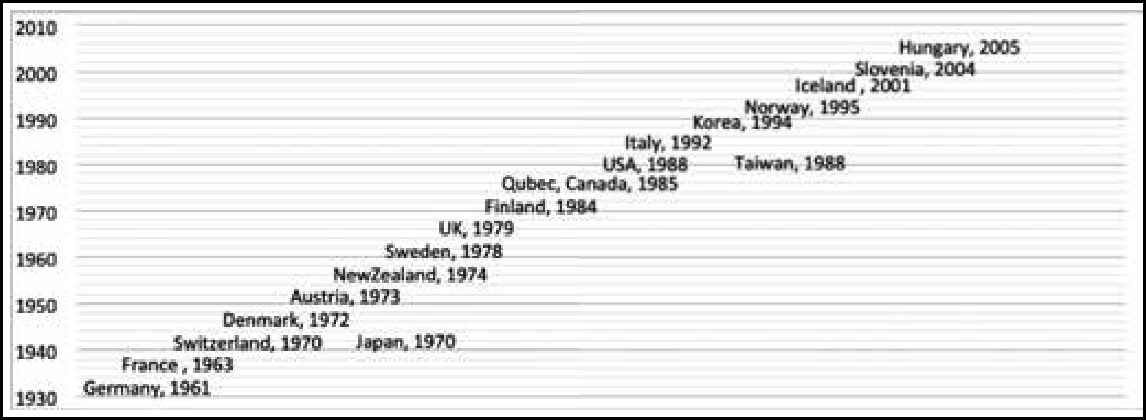
The elements of compensation in these countries include unreimbursed medical costs, disability pension, noneconomic loss, funeral costs, cost of future care, lost wages and death benefits. The eligibility of particular people for compensation and the amount they may receive are decided by the national governments. Some of the factors that determine the eligibility to receive compensation are age, the time-frame within which compensation should be claimed, citizenship status, the location at which the vaccine was administered (public or private establishments), and whether the vaccine is recommended or compulsory.
Some factors that influence whether or not a vaccine is covered under the compensation programme are whether the vaccine is mandatory, or is administered as a part of travel or occupational requirements. The vaccines covered by the compensation mechanism vary across countries, as shown in Figure 2.
| Country | Compensation eligibility |
| USA | Childhood vaccines, vaccines administered to the armed forces, influenza vaccines |
| UK | Childhood vaccines, vaccines administered to the armed forces, influenza vaccines
In instances with more than 60% disability |
| Italy | Injuries from mandatory vaccines, vaccines administered as part of travel or occupational requirement |
| New Zealand | Severe injuries |
| Finland | Loss of functional ability for a minimum of 14 days |
| Germany | Injury that goes beyond a normal post-vaccine reaction; supplemental payments made if disability continues for more than 6 months |
| Denmark | Permanent injury caused by vaccination |
| Quebec, Canada | Permanent physical or mental injury or death caused by vaccination |
| South Korea | Injuries from vaccines included in the National Immunisation Programme
Compensation for medical bills, fixed nursing fee, temporary indemnity for the disabled/deceased, funeral service costs |
| Fig. 2: Eligibility for compensation for vaccine-related injuries under the compensation mechanisms of various countries (5, 6). | |
The source of funds for the payment of compensation is also a matter of debate. The question arises as to whether it should be the government or the manufacturers’ levy paid by the pharmaceutical companies. The source of funding for compensation largely reflects where the decision-making power lies. Several countries finance their programmes from the national, state or municipal treasuries or, as in the case of Japan, a combination of all these. Finland, Norway and Sweden use the manufacturers’ levy to finance compensation. New Zealand’s scheme is financed from several sources, including levies on employers, employees and motor vehicle owners, government funding and investment returns. Taiwan (China) and the USA retain centralised government control over their schemes, which are funded from a vaccine tax. In Taiwan, the manufacturer or importer of the vaccine pays a tax of one New Taiwan dollar (US$ 0.034) per vaccine dose. In the USA, the tax is US$ 0.75 per dose.
In most countries, in general, patients receive primary support from public or private insurers. The compensation schemes can be relatively modest in size and do not need to cover the full range of expenses that might be considered in a tort or product liability case. For example, in Taiwan and the USA, a vaccine tax becomes the corpus for paying compensation for vaccine-related injury. According to the 2016data and statistics report of the US Department of Health and Human Services, a total of 17,437 petitions for compensation had been filed since the inception of the vaccine compensation programme in 1988. On an average, one case of compensation is filed per million vaccinations done. While 10,086 cases were dismissed; compensation was provided in 4954 cases, while the other cases are still being scrutinised. Nearly 3.4 billion dollars have been paid as compensation so far.
The compensation programme in Sweden is nongovernmental and the decisions are not linked with legal proceedings. To curb malpractice in medicine, the Swedish national government started an insurance programme for patients in 1975. This was followed by pharmaceutical insurance, launched by pharmaceutical companies in 1978. As for South Korea, according to the 2013 report of the Korean National Immunisation Programme for Children, a total of 5,372 adverse events were reported from 2002 to 2011. Of the 471 requests for compensation, compensation was granted in 234 cases, while the remaining 237 requests were rejected (3).
As for compensation systems with regard to AEFIs, one of the important factors to consider has been whether there are any causal links between the injury suffered and the vaccine in question. It is very difficult to establish causation in vaccine-related injuries, given the lack of “markers”. There is also a variety of views regarding the mechanisms to be used to probe the element of causation. One of the important epidemiological means to do so is to use the Bradford Hill criteria, which aid in sorting and sifting through observed associations that can be considered causal or non-causal. However, unlike epidemiological means, the mechanisms for establishing causality are different in tort law and other legal instruments. The incisive argument of Looker and Kelly is worth noting in this regard.
In tort litigation the defendant, or defective product, is on trial for ‘causing’ a specific individual’s or group’s adverse outcome. A direct link must be established between the particular action of that defendant or product and the adverse outcome. Legal causation is deterministic and requires proof of an allegation. In general, most compensation schemes offer a more liberal approach to standard of proof than the legal standard. (5)
For example, in the USA, information on the risks and benefits of vaccines is disseminated by the providers of immunisation, who are directed by the law to channel the process through the Centres for Disease Control Vaccine Information Statements (5). For any of the vaccines included in this system, a claim for compensation can be initiated by any individual (or his/her parents, legal guardians, trustees, etc, in the case of children or incapacitated persons) who has suffered injury or death. However, the law requires that the claim should have a demonstrable link to the vaccine in question, with the vaccine being shown to be the causal factor. The types and nature of injuries that can be compensated for are listed in the Vaccine Injury Table of the Code of Federal Regulations, Section 2114 of the National Childhood Vaccine Injury Act. Injuries that are not on the list must be demonstrated to have a causal link with the vaccine and the onus of establishing this lies on the petitioner(s) claiming compensation (4).
However, in many of the countries (Figure 1) which have some mechanism for compensation, provision has been made to extend relief to the injured even before the investigative procedures are completed. This is not the case in India, where those who are affected have to wait till the culmination of legal proceedings under the tort law. In the subsequent sections in this paper, we explore this aspect in some detail.
The Indian context
In India, measures for the surveillance of AEFIs started being taken in 1986.The most recent version is the AEFI Surveillance Guideline of the Government of India (2014). Data and reports on AEFIs can be an important source for assessing injury for the purpose of compensation. However, the question of whether it is also necessary to move beyond the classification of AEFIs requires some thought. Figure 3 presents the classification of AEFIs.
| A1 | Reaction related to vaccine product |
| A2 | Reaction related to vaccine quality defect |
| A3 | Reaction related to immunisation error |
| A4 | Reaction related to immunisation anxiety |
| B1 | Temporal relationship is consistent but there is insufficient definitive evidence that it is the vaccine that has caused the event |
| B2 | Reviewing factors result in conflicting trends of consistency and inconsistency with causal association to immunisation |
| C | Coincidental-underlying or emerging condition(s), or conditions caused by exposure to something other than vaccine |
| D | Unclassifiable |
| Fig. 3: Classification of AEFIs according to MoHF W guideline, 2014 | |
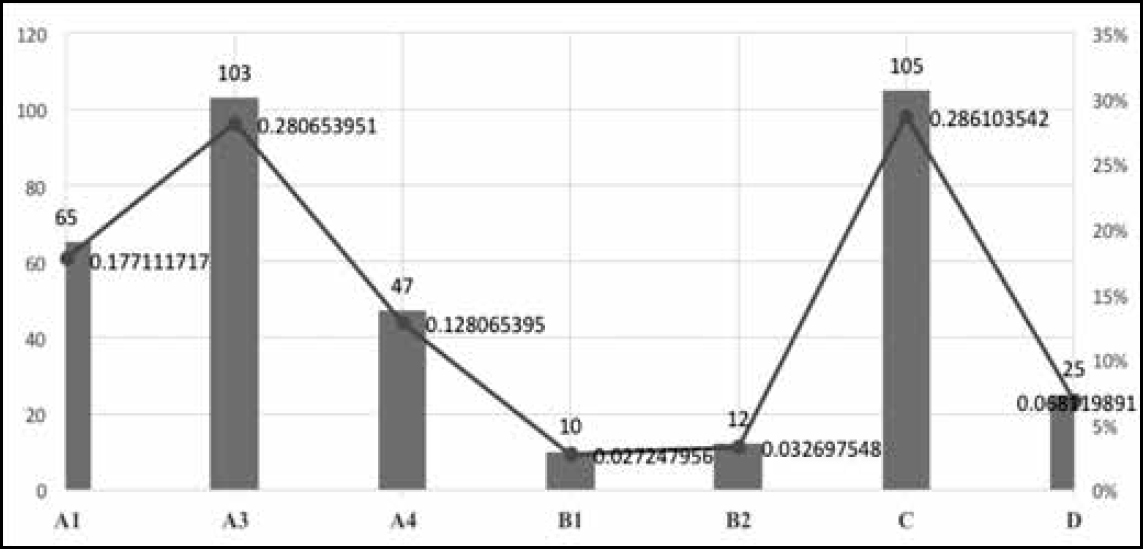
The most important AEFIs reported from among those listed in this classification are A1 and A3, ie “Reaction related to vaccine product” and “Reaction related to immunisation error”. The number of reported cases with a Detailed Immunisation Report, which allows for the assessment of causality, is considered to be an indicator of effective surveillance of AEFIs. In India, a causality assessment is undertaken by a sub-committee entrusted with the task of strengthening the national AEFI surveillance system, under the aegis of the Immunisation Division of the Ministry of Health and Family Welfare (MoHFW). This mode of assessment was modelled on the Causality Assessment Protocol of the World Health Organisation (WHO) and was finally reported to the National AEFI Committee. During 2012–14, the Committee examined and reported 367 cases of serious AEFIs from various states in India. The following are some of the insights gained from a preliminary analysis of these cases.
During the period 2012–14,1346 million doses of antigens were administered and 1759 cases of SAEs were reported. Causality assessment reports were available for 367 of these cases. Among these, the highest number of 105 (28.6%) were classified as “C”, ie coincidental; 103 as “A3”, ie related to immunisation error; and 65 as “A1”, ie related to the vaccine product. Thus, A1 and A3 put together constituted 46% of the total of 367 cases subjected to causality assessment.
Figure 4 shows plots of the distribution of AEFI cases classified according to categories.
Among the 367 SAEs reported from the various states, the maximum number of cases was reported from West Bengal. Eighty of the 84 cases from the state in 2013 were linked to hepatitis B immunisation and the causality was established as immunisation error.
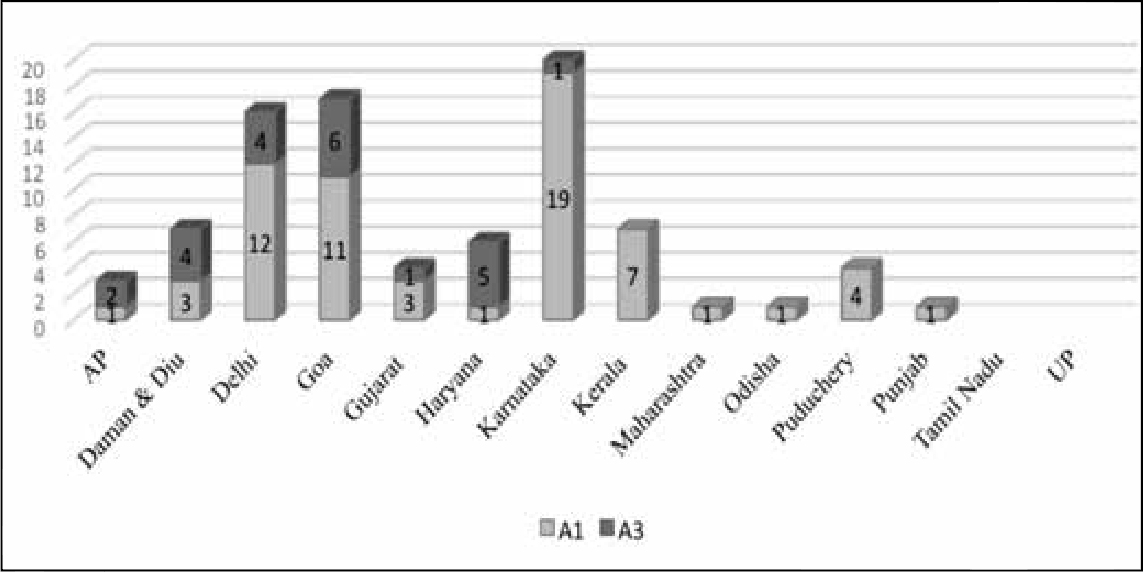
Excluding the 80 cases of immunisation error in West Bengal, Kerala and Bihar had the highest incidence of SAEs. The maximum number of product-related reactions occurred in Kerala, followed by Delhi and Goa. Maharashtra, Punjab and Kerala had the maximum number of immunisation errors. There were very few cases in Uttar Pradesh. However, the low number of cases may be indicative of a lack of effective surveillance or follow-up, rather than the absence of adverse events. Conversely, the high incidence of immunisation error reported from Kerala may be related to strong reporting mechanisms in the state.
In this classification, the most important categories for which compensation must be considered are A1 and A3. Figure 5 depicts the distribution of A1 and A3 cases across the states, according to the report.
On death as a “reason for reporting”
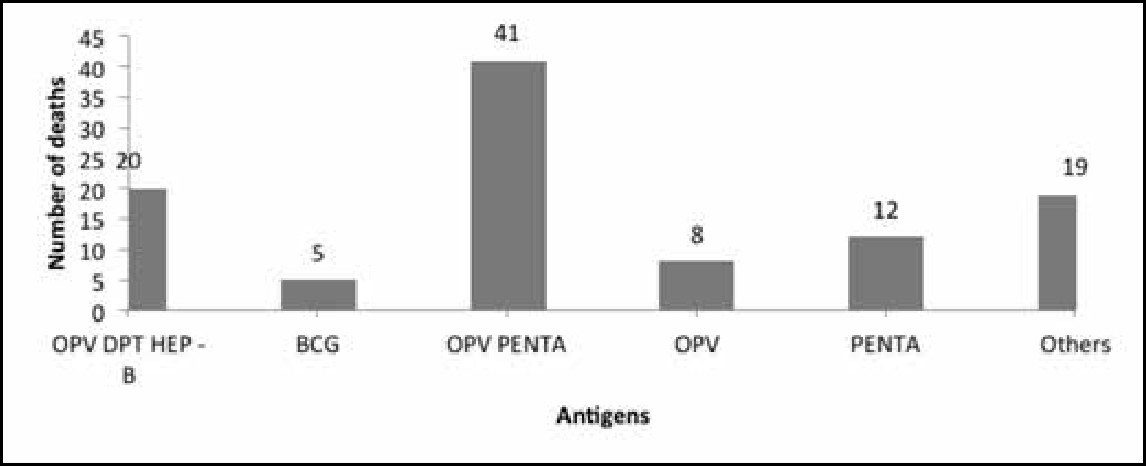
A striking fact that emerges from the causality assessment of the 367 AEFI cases reviewed and approved by the national AEFI committee is that in 105 cases, the reason for reporting was death. Of these cases, 86 were reported after the administration of five antigens (Figure 6): oral polio vaccine (OPV), diphtheria, tetanus and pertussis (DPT), hepatitis B, bacillus Calmette–Guérin (BCG), OPV pentad, OPV and pentavalent.3
It is to be noted that in the data on deaths, the AEFI classification for 65 cases was “C”, ie “coincidental”, and that for 24 cases was “D”, ie “unclassifiable”. Only one case was classified as A1, ie “reaction related to vaccine product” (Figure 7).
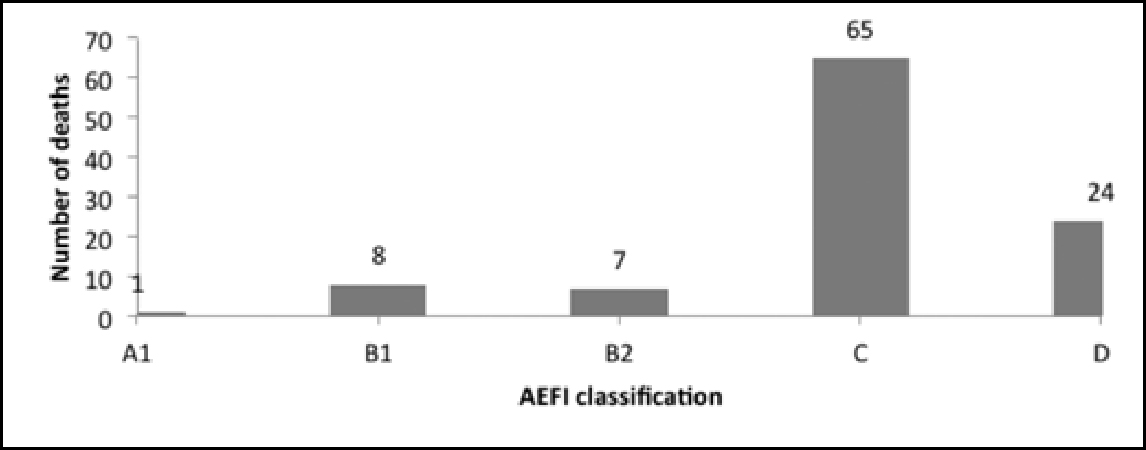
The causality assessment report of the MoHFW states: “Most of the reported serious AEFIs are coincidental. “However, as demonstrated in this section and in Figure 4, the number of cases classified as “coincidental” is equal to the number of cases the reason for reporting which was death, ie 105. The high number of cases designated C and D (Figure 7) warrants further investigation. One must keep in mind the underlying ethical concerns when looking into the fact that deaths are being reported in relation to vaccines, irrespective of the fact that the assessment of causality may indicate no causal association.
Compensation for vaccine injuries in India
India has no official vaccine compensation programme for vaccine-related injuries or deaths. The only option that complainants have is to approach the legal system, which is an expensive and protracted process, ranging at times from 10 to 15 years. Moreover, establishing causality and fault is an extremely challenging task.
In the Dr Durga Nursing Home vs K Dhanasekaran case in 2003 (before the State Consumer Disputes Redressal Commission, Chennai) (7), for example, the petitioner approached the district consumer court, alleging negligence and the use of expired vaccines. The court concluded that the nursing home did not have an effective storage system and its ambulance facility was inadequate, and thus, its services were deficient. It awarded a compensation of INR 100,000 for mental agony and hardship and INR 5000 towards costs. Both parties then appealed in the state consumer court. The latter declared that there was not enough evidence to comment on the quality of the vaccines used, but found that the instruments which were required were not maintained properly and there was no ambulatory service. The court held the hospital liable to pay and ruled in favour of a compensation of INR 300,000 for mental agony and hardship and INR 5000 towards costs. This is an important case that establishes the need to focus on injuries that are attributable to the overall process of vaccine administration, including the facilities for storage and ambulances.
In another case, the state of Gujarat appealed (8) against a compensation of INR 100,000 awarded to a petitioner on the ground that the petitioner had suffered permanent deformity and disability following negligent administration of a triple vaccine. The High Court upheld the appeal, reversed the decree of the trial court and stayed the execution of the money decree. While dismissing the claim, it maintained that the claimant had been unable to establish a causal linkage or the fact that there had been negligence. The decision of the High Court came 15 years after the petitioner had initiated the suit.
In 2013, a civil writ petition was filed in the Supreme Court by Sama: Resource Group for Women and Health and Others against the Union of India and Others (9), demanding compensation for the deaths of seven girls during the “observational study” of HPV vaccines4 by the Programme for Appropriate Technology in Health (PATH). At the time of the submission of this paper, the case was still pending in the Supreme Court and no decision had been taken on compensation.
These cases underscore some critical issues. One is that the tort system places the onus of establishing adverse events on the affected parties, and this consequently has an impact on their claim to compensation. Even countries with established compensation mechanisms have attested to this difficulty inherent in legal mechanisms. They, however, continue to uphold the State’s responsibility towards people who suffer injury or death.
The current scenario in India necessitates the recognition of an injury following vaccination and the formulation of an appropriate compensation policy. In the absence of such a policy, the affected parties will be left with no option other than to approach the legal system under tort law. Considering that the latter is an extremely challenging process, there is a need for a system that goes beyond it and is based on the right of the affected parties to receive comprehensive medical care, as well as compensation, in case of AEFIs.
Conclusion
India can gain a few important insights from the attempts being made by some countries to institutionalise compensation for adverse events following the administration of vaccines.
AEFIs, including death, are not rare and can occur despite the best care. It is possible for people to suffer AEFIs even if full attention is paid to the guidelines for the manufacture, storage and distribution of vaccines, and even if the selection of recipients and the technique of vaccination are appropriate. Since vaccination is a public health intervention, vaccines are administered to all people, of whom healthy children comprise the majority. Given that the notion of preventing disease and safeguarding health – either to protect people from certain diseases or to eradicate these diseases – underlies the administration of vaccines, it is of critical importance to provide complete medical management and compensation in case of AEFIs in general. This necessitates the existence of a clear framework or a mechanism of compensation which transcends the boundaries of a legal remedy that places the onus on the affected person. What is required is a comprehensive system that emphasises stronger and time-bound surveillance, reporting and remedial processes. It will not suffice to change the methodology of investigating AEFIs; it is crucial to make the process of assessment transparent to understand how the investigation is carried out, documented and publicised. One must ascertain whether the affected parties or their families, guardians, etc. are involved in the process; if not, the assessment will be biased and does not follow the principles of natural justice.
Data from the investigation of AEFIs must be placed in the public domain to work towards an ethical and transparent system. Serious consideration must be given to the question of compensation, irrespective of any causal association between the AEFI and the vaccine. Deaths and adverse events following compulsory immunisation must be adequately compensated on the basis of “no fault”.
While further deliberations may be necessary among policymakers and all stakeholders to develop a clear system for compensation, in principle, acceptance of the need for compensation should not be delayed any further. Finally, any compensation mechanism in the context of AEFIs must, besides awarding compensation, emphasise the acknowledgement of a “wrong” or “fault” towards reparation of the affected.
Acknowledgements
The authors would like to acknowledge Dr Yogesh Jain and Mr Ranjan De for their comments and inputs, and Dr Ruchi Bhargava and Ms Megha Kain of Sama for helping with the graphs.
Notes:
1 According to the “Good Clinical Practice Guidelines” of The Central Drugs Standard Control Organisation, an adverse event (AE) is defined as any untoward medical occurrence (including a symptom / disease or an abnormal laboratory finding) which takes place during treatment with a pharmaceutical product in a patient or a human volunteer and which does not necessarily have a relationship with the treatment being given. A serious adverse event is an AE associated with death, inpatient hospitalisation (if the study is being conducted on outpatients), prolongation of hospitalisation (if the study is being conducted on inpatients), persistent or significant disability or incapacity, or a congenital anomaly or birth defect, or is otherwise life-threatening. See: www.cdsco.nic.in/html/GCP1.html
2 According to the Report of CIOMS/WHO Working Group on Vaccine Pharmacovigilance, 2012, an AEFI is any untoward medical occurrence which follows immunisation and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavourable or unintended sign, abnormal laboratory finding, symptom or disease.
3 The other antigens that were linked to the remaining 19 cases of death were: OPV DPT VIT – A, BCG, DPT HEP – B, BOPV BCG, OPV PENTA, DPT VIT – A, MEASLES, OPV, OPV DPT, EASY 4, OPV BCG PENTA, OPV DPT HEP MEASLES BCG DT, OPV DPT MEASLES, OPV DPT HEP – B BCG, OPV HEP – B BCG and OPV DPT BCG.
4 The “observational study” of HPV vaccines was carried out by the Programme for Appropriate Technology in Health, in collaboration with the Andhra Pradesh and Gujarat governments and with funding from the Bill and Melinda Gates Foundation. The vaccines were provided free of cost by the manufacturing companies Merck and GlaxoSmithKline, and the technical support for these “projects” was provided by the Indian Council of Medical Research. The vaccine projects were suspended in 2010.
References
- Oxford English Dictionary [Internet] [cited 2017 Mar 31]. Available from: http://oxforddictionaries.com/definition/english/compensation
- Childress JF. Compensating injured research subjects: I. The moral argument. Hastings Cent Rep. 1976 Dec;6(6):21-7.
- Evans G. Vaccine injury compensation programs worldwide. Vaccine. 1999 Oct 29;17Suppl 3:S25-35.
- Edlich RF, Olson DM, Olson BM, Greene JA, Gubler KD, Winters KL, Kelley AR, Britt LD, Long WB 3rd.Update on the national vaccine injury compensation program. J Emerg Med. 2007 Aug;33(2):199-211. Epub 2007 Jun 18.
- Looker C, Kelly H. No fault compensation following adverse events attributed to vaccination: a review of international programmes [Internet]. Bull World Health Organ. 2011 May 1 [cited 2017 Mar 31];89(5):371-8. doi: 10.2471/BLT.10.081901. Epub 2011 Mar 21. Available from: http://www.who.int/bulletin/volumes/89/5/10-081901/en/.
- Danish Health and Medicines Authority. The Danish Childhood vaccination programme, 2015 [cited 2017 Mar 31]. Available from: https://www.sst.dk/en/disease-and-treatment/~/media/B74655FEA6DF4771998A6BDEA96A374A.ashx
- Dr Durga Nursing Home vs K. Dhanasekharan before the State Consumer Disputes Redressal Commission, Chennai, F.A. Nos. 1070/2011 and 683/2012.
- State of Gujarat and Others vs Shahenazbanu Ashrafali on July 13, 1995. Gujarat High Court. 1997 ACJ 176, AIR 1996 Guj 136 [Internet] [cited 2017 Mar 31]. Available from: https://indiankanoon.org/doc/1026674/
- Sama: Resource Group for Women and Health and others vs Union of India and others (WRIT PETITON (CIVIL) NO 921 OF 2013).